Acetogenin
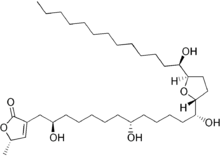
Acetogenins are a class of polyketide natural products found in plants of the family Annonaceae. They are characterized by linear 32- or 34-carbon chains containing oxygenated functional groups including hydroxyls, ketones, epoxides, tetrahydrofurans and tetrahydropyrans. They are often terminated with a lactone or butenolide.[1] Over 400 members of this family of compounds have been isolated from 51 different species of plants.[2]
Examples include:
Biological effects
Acetogenins have been investigated for their potential therapeutic use in treating cancer,[3] but this potential is tempered with concerns about neurotoxicity.[4][5][6][7][8] Well over half of all cancer patients pursue some sort of complementary and alternative medical treatments.[9] Neither purified acetogenins nor crude extracts of the pawpaw or the Brazilian pawpaw (Asimina triloba, Annonaceae) have been approved by the FDA for cancer treatment, but they have exhibited antitumor efficacy both in animal models and in a limited number of clinical studies.[10] There is a lack of rigorously controlled clinical trials, casting doubt of the efficacy of acetogenins.[11]
Both the Pawpaw extract and acetogenins appear to inhibit HIF-1 activation by blocking the hypoxic induction of nuclear HIF-1α protein.[11]
References
- ↑ Li, N.; Shi, Z.; Tang, Y.; Chen, J.; Li, X. (2008). "Recent Progress on the Total Synthesis of Acetogenins from Annonaceae" (PDF). Beilstein Journal of Organic Chemistry. 4 (48): 1–62. doi:10.3762/bjoc.4.48. PMC 2633664
 . PMID 19190742.
. PMID 19190742. - ↑ Bermejo, A.; Figadère, B.; Zafra-Polo, M.-C.; Barrachina, I.; Estornell, E.; Cortes, D. (2005). "Acetogenins from Annonaceae: Recent Progress in Isolation, Synthesis and Mechanisms of Action". Natural Product Reports. 22 (2): 269–303. doi:10.1039/B500186M. PMID 15806200. Erratum: "Back Matter". Natural Product Reports. 22 (3): 426. 2005. doi:10.1039/B503508M.
- ↑ Mangal, M; Khan, M. I.; Agarwal, S. M. (2015). "Acetogenins as Potential Anticancer Agents". Anti-cancer agents in medicinal chemistry. 16 (2): 138–59. doi:10.2174/1871520615666150629101827. PMID 26118710.
- ↑ Ana V. Coria-Téllez, Efigenia Montalvo-Gónzalez, Elhadi M. Yahia, Eva N. Obledo-Vázquez (2016). "Annona muricata: A comprehensive review on its traditional medicinal uses, phytochemicals, pharmacological activities, mechanisms of action and toxicity". Arabian Journal of Chemistry. doi:10.1016/j.arabjc.2016.01.004.
- ↑ Le Ven, J.; Schmitz-Afonso, I.; Touboul, D.; Buisson, D.; Akagah, B.; Cresteil, T.; Lewin, G.; Champy, P. (2011). "Annonaceae fruits and parkinsonism risk: Metabolisation study of annonacin, a model neurotoxin; evaluation of human exposure". Toxicology Letters. 205: S50. doi:10.1016/j.toxlet.2011.05.197.
- ↑ Victor R. Preedy; Ronald Ross Watson; Vinood B. Patel, eds. (2011). Nuts and Seeds in Health and Disease Prevention. Academic Press. pp. 434–435.
- ↑ Robert A. Levine, Kristy M. Richards, Kevin Tran, Rensheng Luo, Andrew L. Thomas, and Robert E. Smith (2015). "Determination of Neurotoxic Acetogenins in Pawpaw (Asimina triloba) Fruit by LC-HRMS". J. Agric. Food Chem. 63 (4): 1053–1056. doi:10.1021/jf504500g.
- ↑ Potts, L. F.; Luzzio, F. A.; Smith, S. C.; Hetman, M; Champy, P; Litvan, I (2012). "Annonacin in Asimina triloba fruit: Implication for neurotoxicity". Neurotoxicology. 33 (1): 53–8. doi:10.1016/j.neuro.2011.10.009. PMID 22130466.
- ↑ Richardson, M. A.; Sanders, T.; Palmer, J. L.; Greisinger, A.; Singletary, S. E. (2000). "Complementary/alternative medicine use in a comprehensive cancer center and the implications for oncology.". Journal of Clinical Oncology. 18 (13): 2505–14. PMID 10893280.
- ↑ McLaughlin, J.L. (2008). "Paw paw and cancer: annonaceous acetogenins from discovery to commercial products.". Journal of Natural Products. 71 (7): 1311–21. doi:10.1021/np800191t. PMID 18598079.
- 1 2 Coothankandaswamy, Veena; Liu, Yang; Mao, Shui-Chun; Morgan, J. Brian; Mahdi, Fakhri; Jekabsons, Mika B.; Nagle, Dale G.; Zhou, Yu-Dong (2010). "The Alternative Medicine Pawpaw and Its Acetogenin Constituents Suppress Tumor Angiogenesis via the HIF-1/VEGF Pathway". Journal of Natural Products. 73 (5): 956–961. doi:10.1021/np100228d. PMC 2890309
 . PMID 20423107.
. PMID 20423107.
External links
-
 Media related to Acetogenins at Wikimedia Commons
Media related to Acetogenins at Wikimedia Commons