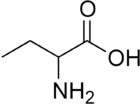
alpha-Aminobutyric acid
 | |
 | |
| Names | |
|---|---|
| IUPAC name
2-Aminobutanoic acid | |
| Other names
2-Aminobutyric acid; α-Aminobutanoic acid; Ethylglycine; Homoalanine | |
| Identifiers | |
| 2835-81-6 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:35621 |
| ChEMBL | ChEMBL55242 |
| ChemSpider | 6405 |
| ECHA InfoCard | 100.018.742 |
| PubChem | 6657 |
| UNII | 8306QPJ19P |
| |
| |
| Properties | |
| C4H9NO2 | |
| Molar mass | 103.12 g/mol |
| Acidity (pKa) | 2.55 (carboxyl), 9.60 (amino)[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
α-Aminobutyric acid (AABA), also known as homoalanine in biochemistry, is a non-proteinogenic alpha amino acid with chemical formula C4H9NO2. The straight two carbon side chain is one carbon longer than alanine, hence the prefix homo-.
Homoalaine is biosynthesised by transaminating oxobutyrate, a metabolite in isoleucine biosynthesis. It is used by nonribosomal peptide synthases. One example of a nonribosomal peptide containing homoalanine is ophthalmic acid, which was first isolated from calf lens.
α-Aminobutyric acid is an isomer of the amino acid aminobutyric acid. It has two other isomers, the neurotransmitter γ-Aminobutyric acid (GABA) and β-Aminobutyric acid (BABA) which is known for inducing plant disease resistance.
References
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
This article is issued from Wikipedia - version of the 10/15/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.