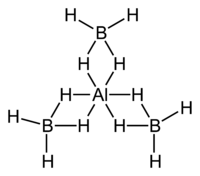
Aluminium borohydride
 | |
| Names | |
|---|---|
| IUPAC name
Aluminium borohydride | |
| Other names
Aluminum borohydride, aluminium tetrahydroborate, aluminum tetrahydroborate | |
| Identifiers | |
| 16962-07-5 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 55734 |
| |
| |
| Properties | |
| AlB3H12 | |
| Molar mass | 71.51 g·mol−1 |
| Appearance | colorless liquid |
| Melting point | −64.5 °C (−84.1 °F; 208.7 K) |
| Boiling point | 44.5 °C (112.1 °F; 317.6 K) |
| reacts | |
| Hazards | |
| Flash point | Spontaneously ignites |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Aluminium borohydride, also known as aluminium tetrahydroborate, (in American English, aluminum borohydride and aluminum tetrahydroborate, respectively) is the chemical compound with the formula Al(BH4)3. It is a volatile pyrophoric liquid which is used as rocket fuel, and as a reducing agent in laboratories. Unlike most other metal–borohydrides, which are ionic structures, aluminium borohydride is a covalent compound.[2][3]
Preparation
Aluminium borohydride is formed by the reaction between sodium borohydride with aluminium chloride:[4]
- 3 NaBH4 + AlCl3 → Al(BH4)3 + 3 NaCl
or as the non-pyrophoric tetrahydrofuran (THF) adduct, by the analogous reaction of calcium borohydride and aluminium chloride in THF:[2]
- 3 Ca(BH4)2 + 2 AlCl3 → 3 CaCl2 + 2 Al(BH4)3
Reactions
Like all borohydrides, this compound is a reducing agent and hydride donor. It reacts with water to give elemental hydrogen gas,[4] and reduces carboxylic esters, aldehydes, and ketones to alcohols.[2]
References
- ↑ Lide, David R. (1998). Handbook of Chemistry and Physics (87 ed.). Boca Raton, FL: CRC Press. pp. 4–39. ISBN 0-8493-0594-2.
- 1 2 3 J. Kollonitsch & O. Fuchs (1955). "Preparation of Aluminium Borohydride and its Applications in Organic Reductions". Nature. 176 (4492): 1081. doi:10.1038/1761081a0.
- ↑ Miwa, K.; Ohba, N.; Towata, S.; Nakamori, Y.; Züttel, A.; Orimo, S. (2007). "First-principles study on thermodynamical stability of metal borohydrides: Aluminum borohydride Al(BH4)3". J. Alloys Compd. 446–447: 310–314. doi:10.1016/j.jallcom.2006.11.140.
- 1 2 Perry, Dale L.; Phillips, Sidney L. (1995). Handbook of Inorganic Compounds. CRC Press. pp. 3–4. ISBN 0-8493-8671-3. Retrieved 2007-12-09.
Further reading
- Fletcher, Edward; Foster, Hampton; Straight, David (1959). "Aluminum Borohydride and Mixtures with Hydrocarbons in Jet Engine Combustor Ignition". Industrial & Engineering Chemistry. 51 (11): 1389. doi:10.1021/ie50599a044.
- Hinkamp, James B.; Hnizda, Vincent (1955). "Aluminum Borohydride Preparation". Industrial & Engineering Chemistry. 47 (8): 1560. doi:10.1021/ie50548a032.