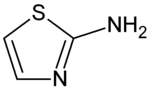
Aminothiazole
 | |
 | |
| Names | |
|---|---|
| IUPAC name
1,3-thiazol-2-amine | |
| Other names
2-Thiazolamine, Aminothiazole, 2-Thiazylamine, Basedol, 2-Thiazolylamine, 4-Thiazolin-2-onimine, 2-Amino-1,3-thiazole, Abadole | |
| Identifiers | |
| 96-50-4 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:40782 |
| ChEMBL | ChEMBL344760 |
| ChemSpider | 2070 |
| ECHA InfoCard | 100.002.284 |
| KEGG | D02479 |
| PubChem | 2155 |
| UNII | 5K8WKN668K |
| |
| |
| Properties | |
| C3H4N2S | |
| Molar mass | 100.14 g·mol−1 |
| Appearance | light yellow crystals |
| Melting point | 86 to 89 °C (187 to 192 °F; 359 to 362 K) |
| Boiling point | 117 °C (243 °F; 390 K) (20 hPa) |
| 100 g/l (20 °C) | |
| Hazards | |
| NFPA 704 | |
| 600 °C (1,112 °F; 873 K) | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
2-Aminothiazole is a heterocyclic amine featuring an thiazole core. It can also be considered a cyclic isothiourea. It possesses an odor similar to pyridine and is soluble in water, alcohols and diethyl ether. It is commonly used as a starting point for the synthesis of many compounds including sulfur drugs, biocides, fungicides, dyes and chemical reaction accelerators. 2-Aminothiazole can be used as a thyroid inhibitor in the treatment of hyperthyroidism and has antibacterial activity. Alternatively, its acid tartrate salt can be used. Recent studies using prion-infected neuroblastoma cell lines have suggested that aminothiazole may be used as a therapeutic drug for prion diseases.[1]
One known use of 2-Aminothiazole in is in the synthesis of Vosaroxin.
References
- ↑ Gallardo-Godoy A; Gever J; Fife KL; Silber BM; Prusiner SB; Renslo AR. (Feb 24, 2011). "2-Aminothiazoles as therapeutic leads for prion diseases.". J Med Chem. 54 (4): 1010–21. doi:10.1021/jm101250y. PMC 3041857
 . PMID 21247166.
. PMID 21247166.
