Amygdala
| Amygdala | |
|---|---|
|
Location of the amygdalae in the human brain | |
|
Subdivision of the amygdala | |
| Details | |
| Identifiers | |
| Latin | corpus amygdaloideum |
| MeSH | Amygdala |
| NeuroNames | hier-219 |
| NeuroLex ID | Amygdala |
| TA | A14.1.09.402 |
| FMA | 61841 |
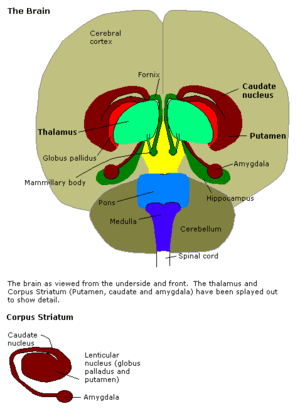
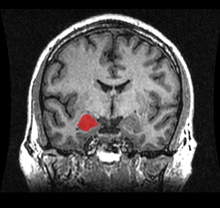
The amygdalae (singular: amygdala; /əˈmɪɡdələ/; also corpus amygdaloideum; Latin, from Greek ἀμυγδαλή, amygdalē, 'almond', 'tonsil'[1]) are two almond-shaped groups of nuclei located deep and medially within the temporal lobes of the brain in complex vertebrates, including humans.[2] Shown in research to perform a primary role in the processing of memory, decision-making, and emotional reactions, the amygdalae are considered part of the limbic system.[3]
Structure
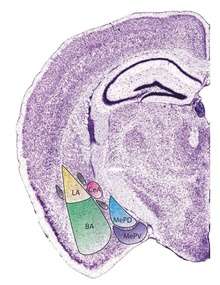
The regions described as amygdala nuclei encompass several structures with distinct connectional and functional characteristics in humans and other animals.[4] Among these nuclei are the basolateral complex, the cortical nucleus, the medial nucleus, the central nucleus, and the intercalated cell clusters (ITCs). The basolateral complex can be further subdivided into the lateral, the basal, and the accessory basal nuclei.[3][5][6]
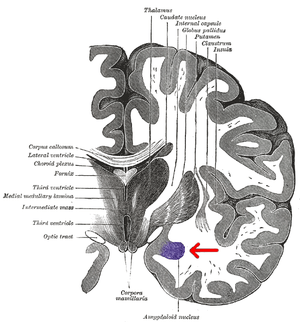
Anatomically, the amygdala[7] and more particularly, its central and medial nuclei,[8] have sometimes been classified as a part of the basal ganglia.
Hemispheric specializations
There are functional differences between the right and left amygdala. In one study, electrical stimulations of the right amygdala induced negative emotions, especially fear and sadness. In contrast, stimulation of the left amygdala was able to induce either pleasant (happiness) or unpleasant (fear, anxiety, sadness) emotions.[9] Other evidence suggests that the left amygdala plays a role in the brain's reward system.[10]
Each side holds a specific function in how we perceive and process emotion. The right and left portions of the amygdala have independent memory systems, but work together to store, encode, and interpret emotion.
The right hemisphere is associated with negative emotion. It plays a role in the expression of fear and in the processing of fear-inducing stimuli. Fear conditioning, which is when a neutral stimulus acquires aversive properties, occurs within the right hemisphere. When an individual is presented with a conditioned, aversive stimulus, it is processed within the right amygdala, producing an unpleasant or fearful response. This emotional response conditions the individual to avoid fear-inducing stimuli.
The right hemisphere is also linked to declarative memory, which consists of facts and information from previously experienced events and must be consciously recalled. It also plays a significant role in the retention of episodic memory. Episodic memory consists of the autobiographical aspects of memory, permitting you to recall your personal emotional and sensory experience of an event. This type of memory does not require conscious recall. The right amygdala plays a role in the association of time and places with emotional properties.[11]
Amygdalar development
There is considerable growth within the first few years of structural development in both male and female amygdalae.[12] Within this early period, female limbic structures grow at a more rapid pace than do males. Amongst female subjects, the amygdala reaches its full growth potential approximately 1.5 years before the peak of male development. The structural development of the male amygdala occurs over a longer period than in women. Despite the early development of female amygdalae, they reach their growth potential sooner than males, whose amygdalae continue to develop. The larger relative size of the male amygdala may be attributed to this extended developmental period.
In addition to longer periods of development, other neurological and hormonal factors may contribute to sex-specific developmental differences. The amygdala is rich in androgen receptors – nuclear receptors that bind to testosterone. Androgen receptors play a role in the DNA binding that regulates gene expression. Though testosterone is present within the female hormonal systems, women have lower levels of testosterone than men. The abundance of testosterone in the male hormonal system may contribute to development. In addition, the grey matter volume on the amygdala is predicted by testosterone levels, which may also contribute to the increased mass of the male amygdala.
In addition to sex differences, there are observable developmental differences between the right and left amygdala in both males and females. The left amygdala reaches its developmental peak approximately 1.5–2 years prior to the right amygdala. Despite the early growth of the left amygdala, the right increases in volume for a longer period of time. The right amygdala is associated with response to fearful stimuli as well as face recognition. It is inferred that the early development of the left amygdala functions to provide infants the ability to detect danger.[12] In childhood, the amygdala is found to react differently to same-sex versus opposite-sex individuals. This reactivity decreases until a person enters adolescence, where it increases dramatically at puberty.[13]
Gender distinction
The amygdala is one of the best-understood brain regions with regard to differences between the sexes. The amygdala is larger in males than females in children ages 7–11,[14] in adult humans,[15] and in adult rats.[16]
In addition to size, other differences between men and women exist with regards to the amygdala. Subjects' amygdala activation was observed when watching a horror film and subliminal stimuli. The results of the study showed a different lateralization of the amygdala in men and women. Enhanced memory for the film was related to enhanced activity of the left, but not the right, amygdala in women, whereas it was related to enhanced activity of the right, but not the left, amygdala in men.[17] One study found evidence that on average, women tend to retain stronger memories for emotional events than men.[18]
The right amygdala is also linked with taking action as well as being linked to negative emotions,[19] which may help explain why males tend to respond to emotionally stressful stimuli physically. The left amygdala allows for the recall of details, but it also results in more thought rather than action in response to emotionally stressful stimuli, which may explain the absence of physical response in women.
Function
Connections
The amygdala sends projections to the hypothalamus, the dorsomedial thalamus, the thalamic reticular nucleus, the nuclei of the trigeminal nerve and the facial nerve, the ventral tegmental area, the locus coeruleus, and the laterodorsal tegmental nucleus.[5]
The medial nucleus is involved in the sense of smell and pheromone-processing. It receives input from the olfactory bulb and olfactory cortex.[20] The lateral amygdalae, which send impulses to the rest of the basolateral complexes and to the centromedial nuclei, receive input from the sensory systems. The centromedial nuclei are the main outputs for the basolateral complexes, and are involved in emotional arousal in rats and cats.[5][6][21]
Emotional learning
In complex vertebrates, including humans, the amygdalae perform primary roles in the formation and storage of memories associated with emotional events. Research indicates that, during fear conditioning, sensory stimuli reach the basolateral complexes of the amygdalae, particularly the lateral nuclei, where they form associations with memories of the stimuli. The association between stimuli and the aversive events they predict may be mediated by long-term potentiation,[22][23] a sustained enhancement of signaling between affected neurons.[24] There have been studies that show that damage to the amygdala can interfere with memory that is strengthened by emotion. One study examined a patient with bilateral degeneration of the amygdala. He was told a violent story accompanied by matching pictures and was observed based on how much he could recall from the story. The patient had less recollection of the story than patients with functional amygdala, showing that the amygdala has a strong connection with emotional learning.[25]
Emotional memories are thought to be stored in synapses throughout the brain. Fear memories, for example, are considered to be stored in the neuronal connections from the lateral nuclei to the central nucleus of the amygdalae and the bed nuclei of the stria terminalis (part of the extended amygdala). Of course, these connections are the not the sole site of fear memories given that the nuclei of the amygdala receive and send information to other brain regions that are important for memory such as the hippocampus. Some sensory neurons project their axon terminals to the central nucleus.[26] The central nuclei are involved in the genesis of many fear responses such as defensive behavior (freezing or escape responses), autonomic nervous system responses (changes in blood pressure and heart rate/tachycardia), neuroendocrine responses (stress-hormone release), etc. Damage to the amygdalae impairs both the acquisition and expression of Pavlovian fear conditioning, a form of classical conditioning of emotional responses.[24]
The amygdalae are also involved in appetitive (positive) conditioning. It seems that distinct neurons respond to positive and negative stimuli, but there is no clustering of these distinct neurons into clear anatomical nuclei.[27][28] However, lesions of the central nucleus in the amygdala have been shown to reduce appetitive learning in rats. Lesions of the basolateral regions do not exhibit the same effect.[29] Research like this indicates that different nuclei within the amygdala have different functions in appetitive conditioning.[30][31] Nevertheless, we found an example of appetitive emotional learning showing an important role for the basolateral amygdala: The naïve female mice are innately attracted to non-volatile pheromones contained in male-soiled bedding, but not by the male-derived volatiles, become attractive if associated with non-volatile attractive pheromones, which act as unconditioned stimulus in a case of Pavlovian associative learning.[32] In the vomeronasal, olfactory and emotional systems, Fos protein show that non-volatile pheromones stimulate the vomeronasal system, whereas air-borne volatiles activate only the olfactory system. Thus, the acquired preference for male-derived volatiles reveals an olfactory-vomeronasal associative learning. Moreover, the reward system is differentially activated by the primary pheromones and secondarily attractive odorants. Exploring the primary attractive pheromone activates the basolateral amygdala and the shell of nucleus accumbens but neither the ventral tegmental area nor the orbitofrontal cortex. In contrast, exploring the secondarily attractive male-derived odorants involves activation of a circuit that includes the basolateral amygdala, prefrontal cortex and ventral tegmental area. Therefore, the basolateral amygdala stands out as the key center for vomeronasal-olfactory associative learning.[33]
Memory modulation
The amygdala is also involved in the modulation of memory consolidation. Following any learning event, the long-term memory for the event is not formed instantaneously. Rather, information regarding the event is slowly assimilated into long-term (potentially lifelong) storage over time, possibly via long-term potentiation. Recent studies suggest that the amygdala regulates memory consolidation in other brain regions. Also, fear conditioning, a type of memory that is impaired following amygdala damage, is mediated in part by long-term potentiation.[22][23]
During the consolidation period, the memory can be modulated. In particular, it appears that emotional arousal following the learning event influences the strength of the subsequent memory for that event. Greater emotional arousal following a learning event enhances a person's retention of that event. Experiments have shown that administration of stress hormones to mice immediately after they learn something enhances their retention when they are tested two days later.[34]
The amygdala, especially the basolateral nuclei, are involved in mediating the effects of emotional arousal on the strength of the memory for the event, as shown by many laboratories including that of James McGaugh. These laboratories have trained animals on a variety of learning tasks and found that drugs injected into the amygdala after training affect the animals' subsequent retention of the task. These tasks include basic classical conditioning tasks such as inhibitory avoidance, where a rat learns to associate a mild footshock with a particular compartment of an apparatus, and more complex tasks such as spatial or cued water maze, where a rat learns to swim to a platform to escape the water. If a drug that activates the amygdalae is injected into the amygdalae, the animals had better memory for the training in the task.[35] If a drug that inactivates the amygdalae is injected, the animals had impaired memory for the task.
Buddhist monks who do compassion meditation have been shown to modulate their amygdala, along with their temporoparietal junction and insula, during their practice.[36] In an fMRI study, more intensive insula activity was found in expert meditators than in novices.[37] Increased activity in the amygdala following compassion-oriented meditation may contribute to social connectedness.[38]
Amygdala activity at the time of encoding information correlates with retention for that information. However, this correlation depends on the relative "emotionalness" of the information. More emotionally arousing information increases amygdalar activity, and that activity correlates with retention. Amygdala neurons show various types of oscillation during emotional arousal, such as theta activity. These synchronized neuronal events could promote synaptic plasticity (which is involved in memory retention) by increasing interactions between neocortical storage sites and temporal lobe structures involved in declarative memory.[39]
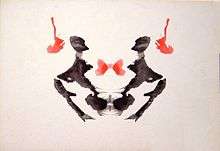
Research using Rorschach test blot 03 finds that the number of unique responses to this random figure links to larger sized amygdalae. The researchers note, "Since previous reports have indicated that unique responses were observed at higher frequency in the artistic population than in the nonartistic normal population, this positive correlation suggests that amygdalar enlargement in the normal population might be related to creative mental activity."[40]
Neuropsychological correlates of amygdala activity
Early research on primates provided explanations as to the functions of the amygdala, as well as a basis for further research. As early as 1888, rhesus monkeys with a lesioned temporal cortex (including the amygdala) were observed to have significant social and emotional deficits.[41] Heinrich Klüver and Paul Bucy later expanded upon this same observation by showing that large lesions to the anterior temporal lobe produced noticeable changes, including overreaction to all objects, hypoemotionality, loss of fear, hypersexuality, and hyperorality, a condition in which inappropriate objects are placed in the mouth. Some monkeys also displayed an inability to recognize familiar objects and would approach animate and inanimate objects indiscriminately, exhibiting a loss of fear towards the experimenters. This behavioral disorder was later named Klüver-Bucy syndrome accordingly,[42] and later research proved it was specifically due to amygdala lesions. Monkey mothers who had amygdala damage showed a reduction in maternal behaviors towards their infants, often physically abusing or neglecting them.[43] In 1981, researchers found that selective radio frequency lesions of the whole amygdala caused Klüver-Bucy syndrome.[44]
With advances in neuroimaging technology such as MRI, neuroscientists have made significant findings concerning the amygdala in the human brain. A variety of data shows the amygdala has a substantial role in mental states, and is related to many psychological disorders. Some studies have shown children with anxiety disorders tend to have a smaller left amygdala. In the majority of the cases, there was an association between an increase in the size of the left amygdala with the use of SSRIs (antidepressant medication) or psychotherapy. The left amygdala has been linked to social anxiety, obsessive and compulsive disorders, and post traumatic stress, as well as more broadly to separation and general anxiety.[45] In a 2003 study, subjects with borderline personality disorder showed significantly greater left amygdala activity than normal control subjects. Some borderline patients even had difficulties classifying neutral faces or saw them as threatening.[46] Individuals with psychopathy show reduced autonomic responses, relative to comparison individuals, to instructed fear cues.[47] In 2006, researchers observed hyperactivity in the amygdala when patients were shown threatening faces or confronted with frightening situations. Patients with severe social phobia showed a correlation with increased response in the amygdala.[48] Similarly, depressed patients showed exaggerated left amygdala activity when interpreting emotions for all faces, and especially for fearful faces. Interestingly, this hyperactivity was normalized when patients were administered antidepressant medication.[49] By contrast, the amygdala has been observed to respond differently in people with bipolar disorder. A 2003 study found that adult and adolescent bipolar patients tended to have considerably smaller amygdala volumes and somewhat smaller hippocampal volumes.[50] Many studies have focused on the connections between the amygdala and autism.[51]
Studies in 2004 and 2006 showed that normal subjects exposed to images of frightened faces or faces of people from another race will show increased activity of the amygdala, even if that exposure is subliminal.[52][53] However, the amygdala is not necessary for the processing of fear-related stimuli, since persons in whom it is bilaterally damaged show rapid reactions to fearful faces, even in the absence of a functional amygdala.[54]
Recent research suggests that parasites, in particular toxoplasma, form cysts in the brain of rats, often taking up residence in the amygdala. This may provide clues as to how specific parasites may contribute to the development of disorders, including paranoia.[55]
Future studies have been proposed to address the role of the amygdala in positive emotions, and the ways in which the amygdala networks with other brain regions.[56]
Sexual orientation
Recent studies have suggested possible correlations between brain structure, including differences in hemispheric ratios and connection patterns in the amygdala, and sexual orientation. Homosexual men tend to exhibit more feminine patterns in the amygdala than heterosexual males do, just as homosexual females tend to show more masculine patterns in the amygdala than heterosexual women do. It was observed that amygdala connections were more widespread from the left amygdala in homosexual males, as is also found in heterosexual females. Amygdala connections were more widespread from the right amygdala in homosexual females, as in heterosexual males.[57][58]
Social interaction
Amygdala volume correlates positively with both the size (the number of contacts a person has) and the complexity (the number of different groups to which a person belongs) of social networks.[59][60] Individuals with larger amygdalae had larger and more complex social networks. They were also better able to make accurate social judgments about other persons' faces.[61] The amygdala's role in the analysis of social situations stems specifically from its ability to identify and process changes in facial features. It does not, however, process the direction of the gaze of the person being perceived.[62][63]
The amygdala is also thought to be a determinant of the level of a person's emotional intelligence. It is particularly hypothesized that larger amygdalae allow for greater emotional intelligence, enabling greater societal integration and cooperation with others.[64]
The amygdala processes reactions to violations concerning personal space. These reactions are absent in persons in whom the amygdala is damaged bilaterally.[65] Furthermore, the amygdala is found to be activated in fMRI when people observe that others are physically close to them, such as when a person being scanned knows that an experimenter is standing immediately next to the scanner, versus standing at a distance.[65]
Aggression
Animal studies have shown that stimulating the amygdala appears to increase both sexual and aggressive behavior. Likewise, studies using brain lesions have shown that harm to the amygdala may produce the opposite effect. Thus, it appears that this part of the brain may play a role in the display and modulation of aggression.[66]
Fear
There are cases of human patients with focal bilateral amygdala lesions, due to the rare genetic condition Urbach-Wiethe disease.[67][68] Such patients fail to exhibit fear-related behaviors, leading one, Patient S.M., to be dubbed the "woman with no fear". This finding reinforces the conclusion that the amygdala "plays a pivotal role in triggering a state of fear".[69]
Alcoholism and binge drinking
The amygdala appears to play a role in binge drinking, being damaged by repeated episodes of intoxication and withdrawal.[70] Alcoholism is associated with dampened activation in brain networks responsible for emotional processing, including the amygdala.[71] Protein kinase C-epsilon in the amygdala is important for regulating behavioral responses to morphine, ethanol, and controlling anxiety-like behavior. The protein is involved in controlling the function of other proteins and plays a role in development of the ability to consume a large amount of ethanol.[72][73]
Anxiety
There may also be a link between the amygdala and anxiety.[74] In particular, there is a higher prevalence of females that are affected by anxiety disorders. In an experiment, degu pups were removed from their mother but allowed to hear her call. In response, the males produced increased serotonin receptors in the amygdala but females lost them. This led to the males being less affected by the stressful situation.
The clusters of the amygdala are activated when an individual expresses feelings of fear or aggression. This occurs because the amygdala is the primary structure of the brain responsible for flight or fight response. Anxiety and panic attacks can occur when the amygdala senses environmental stressors that stimulate fight or flight response.
The amygdala is directly associated with conditioned fear. Conditioned fear is the framework used to explain the behavior produced when an originally neutral stimulus is consistently paired with a stimulus that evokes fear. The amygdala represents a core fear system in the human body, which is involved in the expression of conditioned fear. Fear is measured by changes in autonomic activity including increased heart rate, increased blood pressure, as well as in simple reflexes such as flinching or blinking.
The central nucleus of the amygdala has direct correlations to the hypothalamus and brainstem – areas directly related to fear and anxiety. This connection is evident from studies of animals that have undergone amygdalae removal. Such studies suggest that animals lacking an amygdala have less fear expression and indulge in non-species-like behavior. Many projection areas of the amygdala are critically involved in specific signs that are used to measure fear and anxiety.
Mammals have very similar ways of processing and responding to danger. Scientists have observed similar areas in the brain – specifically in the amygdala – lighting up or becoming more active when a mammal is threatened or beginning to experience anxiety. Similar parts of the brain are activated when rodents and when humans observe a dangerous situation, the amygdala playing a crucial role in this assessment. By observing the amygdala’s functions, people can determine why one rodent may be much more anxious than another. There is a direct relationship between the activation of the amygdala and the level of anxiety the subject feels.
Feelings of anxiety start with a catalyst – an environmental stimulus that provokes stress. This can include various smells, sights, and internal feelings that result in anxiety. The amygdala reacts to this stimuli by preparing to either stand and fight or to turn and run. This response is triggered by the release of adrenaline into the bloodstream. Consequently, blood sugar rises, becoming immediately available to the muscles for quick energy. Shaking may occur in an attempt to return blood to the rest of the body. A better understanding of the amygdala and its various functions may lead to a new way of treating clinical anxiety.[75]
Posttraumatic stress disorder
There seems to be a connection with the amygdalae and how the brain processes posttraumatic stress disorder. Multiple studies have found that the amygdalae may be responsible for the emotional reactions of PTSD patients. One study in particular found that when PTSD patients are shown pictures of faces with fearful expressions, their amygdalae tended to have a higher activation than someone without PTSD.[76]
Bipolar disorder
Amygdala dysfunction during face emotion processing is well-documented in bipolar disorder. Individuals with bipolar disorder showed greater amygdala activity (especially the amygdala/medial-prefrontal-cortex circuit).[77] [78]
Political orientation
Amygdala size has been correlated with cognitive styles with regard to political thinking. A study found that "greater liberalism was associated with increased gray matter volume in the anterior cingulate cortex, whereas greater conservatism was associated with increased volume of the right amygdala."[79]
See also
- Amygdala hijack
- BELBIC
- List of regions in the human brain
- Triune brain
- Intercalated cells of the amygdala
Further reading
- Amygdala Joseph E. LeDoux, Scholarpedia, 3(4):2698. doi:10.4249/scholarpedia.2698
References
- ↑ "Amygdala - Define Amygdala at Dictionary.com". Retrieved 9 November 2016.
- ↑ University of Idaho College of Science (2004). "amygdala". Archived from the original on 31 March 2007. Retrieved 15 March 2007.
- 1 2 Amunts K, Kedo O, Kindler M, Pieperhoff P, Mohlberg H, Shah N, Habel U, Schneider F, Zilles K (2005). "Cytoarchitectonic mapping of the human amygdala, hippocampal region and entorhinal cortex: intersubject variability and probability maps". Anat Embryol (Berl). 210 (5–6): 343–52. doi:10.1007/s00429-005-0025-5. PMID 16208455.
- ↑ Bzdok D, Laird A, Zilles K, Fox PT, Eickhoff S.: An investigation of the structural, connectional and functional sub-specialization in the human amygdala. Human Brain Mapping, 2012.
- 1 2 3 Ben Best (2004). "The Amygdala and the Emotions". Archived from the original on 9 March 2007. Retrieved 15 March 2007.
- 1 2 Solano-Castiella E, Anwander A, Lohmann G, Weiss M, Docherty C, Geyer S, Reimer E, Friederici AD, Turner R (2010). "Diffusion tensor imaging segments the human amygdala in vivo". NeuroImage. 49 (4): 2958–65. doi:10.1016/j.neuroimage.2009.11.027. PMID 19931398.
- ↑ See Amygdala in the BrainInfo database
- ↑ Larry W. Swanson; Gorica D. Petrovich (August 1998). "What is the amygdala?". Trends in Neurosciences. 21 (8): 323–331. doi:10.1016/S0166-2236(98)01265-X.
- ↑ Lanteaume, L.; et al. (Jun 2007). "Emotion induction after direct intracerebral stimulations of human amygdala". Cerebral Cortex. 17 (6): 1307–13. doi:10.1093/cercor/bhl041. PMID 16880223.
- ↑ Murray, Elizabeth A.; et al. (2009). "Amygdala function in positive reinforcement". The Human Amygdala. Guilford Press.
- ↑ Markowitsch, H. (1998). Differential contribution of right and left amygdala to affective information processing. IOS Press. 11(4), 233–244.
- 1 2 Uematsu, A., Matsui, M., Tanaka C., Takahashi, T., Noguchi K., Suzuki M., Nishijo H. (2012). Developmental trajectories of amygdala and hippocampus from infancy to early adulthood in healthy individuals. PLOS One Journal. doi:10.1371/journal.pone.0046970
- ↑ Telzer, E. H., Flannery, J., Humphreys, K. L., Goff, B., Gabard-Durman, L., Gee, D. G., & Tottenham, N. (2015). 'The cooties effect': Amygdala reactivity to opposite- versus same-sex faces declines from childhood to adolescence. Journal Of Cognitive Neuroscience, 27(9), 1685-1696. doi:10.1162/jocn_a_00813
- ↑ Caviness, V. S.; Kennedy, D. N.; Richelme, C.; Rademacher, J.; Filipek, P. A. (1996). "The Human Brain Age 7–11 Years: A Volumetric Analysis Based on Magnetic Resonance Images". Cerebral Cortex. 6 (5): 726–36. doi:10.1093/cercor/6.5.726. PMID 8921207.
- ↑ Goldstein, J. M.; Seidman, LJ; Horton, NJ; Makris, N; Kennedy, DN; Caviness Jr, VS; Faraone, SV; Tsuang, MT (2001). "Normal Sexual Dimorphism of the Adult Human Brain Assessed by in Vivo Magnetic Resonance Imaging". Cerebral Cortex. 11 (6): 490–7. doi:10.1093/cercor/11.6.490. PMID 11375910.
- ↑ Hines, Melissa; Allen, Laura S.; Gorski, Roger A. (1992). "Sex differences in subregions of the medial nucleus of the amygdala and the bed nucleus of the stria terminalis of the rat". Brain Research. 579 (2): 321–6. doi:10.1016/0006-8993(92)90068-K. PMID 1352729.
- ↑ Cahill, L; Haier, RJ; White, NS; Fallon, J; Kilpatrick, L; Lawrence, C; Potkin, SG; Alkire, MT (2001). "Sex-Related Difference in Amygdala Activity during Emotionally Influenced Memory Storage". Neurobiology of Learning and Memory. 75 (1): 1–9. doi:10.1006/nlme.2000.3999. PMID 11124043.
- ↑ Hamann, Stephan (2005). "Sex Differences in the Responses of the Human Amygdala". Neuroscience. 11 (4): 288–93. doi:10.1177/1073858404271981. PMID 16061516.
- ↑ Lanteaume, L.; Khalfa, S.; Régis, J.; Marquis, P.; Chauvel, P.; Bartolomei, F. (2006). "Emotion Induction After Direct Intracerebral Stimulations of Human Amygdala". Cerebral Cortex. 17 (6): 1307–13. doi:10.1093/cercor/bhl041. PMID 16880223.
- ↑ Carlson, Neil (12 January 2012). Physiology of behavior. Pearson. p. 336. ISBN 978-0205239399.
- ↑ Groshek, Frank; Kerfoot, Erin; McKenna, Vanessa; Polackwich, Alan S.; Gallagher, Michela; Holland, Peter C. (2005). "Amygdala Central Nucleus Function is Necessary for Learning, but Not Expression, of Conditioned Auditory Orienting". Behavioral Neuroscience. 119 (1): 202–12. doi:10.1037/0735-7044.119.1.202. PMC 1255918
 . PMID 15727525.
. PMID 15727525. - 1 2 Maren (Dec 1999). "Long-term potentiation in the amygdala: a mechanism for emotional learning and memory". Trends Neurosci. 22 (12): 561–7. doi:10.1016/S0166-2236(99)01465-4. PMID 10542437.
- 1 2 Blair, H. T. (2001). "Synaptic Plasticity in the Lateral Amygdala: A Cellular Hypothesis of Fear Conditioning". Learning & Memory. 8 (5): 229–242. doi:10.1101/lm.30901.
- 1 2 Ressler, Kerry; Davis, Michael (2003). "Genetics of Childhood Disorders: L. Learning and Memory, Part 3: Fear Conditioning". Journal of the American Academy of Child & Adolescent Psychiatry. 42 (5): 612–5. doi:10.1097/01.CHI.0000046835.90931.32. PMID 12707566.
- ↑ Carlson, Neil R. (12 January 2012). Physiology of Behavior. Pearson. p. 364. ISBN 978-0205239399.
- ↑ Carlson, Neil R. (12 January 2012). Physiology of Behavior. Pearson. p. 453. ISBN 978-0205239399.
- ↑ Paton, Joseph J.; Belova, Marina A.; Morrison, Sara E.; Salzman, C. Daniel (2006). "The primate amygdala represents the positive and negative value of visual stimuli during learning". Nature. 439 (7078): 865–70. doi:10.1038/nature04490. PMC 2396495
 . PMID 16482160.
. PMID 16482160. - ↑ Redondo, RL; Kim, J; Arons, AL; Ramirez, S; Liu, X; Tonegawa, S (2014). "Bidirectional switch of the valence associated with a hippocampal contextual memory engram". Nature. 513: 426–30. doi:10.1038/nature13725.
- ↑ Parkinson, John A.; Robbins, Trevor W.; Everitt, Barry J. (2000). "Dissociable roles of the central and basolateral amygdala in appetitive emotional learning". European Journal of Neuroscience. 12 (1): 405–13. doi:10.1046/j.1460-9568.2000.00960.x. PMID 10651899.
- ↑ See recent TINS article by Balleine and Killcross (2006)
- ↑ Killcross S, Robbins T, Everitt B (1997). "Different types of fear-conditioned behaviour mediated by separate nuclei within amygdala". Nature. 388 (6640): 377–80. doi:10.1038/41097. PMID 9237754.
- ↑ Moncho-Bogani J, Lanuza E, Hernández A, Novejarque A, Martínez-García F. Attractive properties of sexual pheromones in mice: innate or learned? Physiol Behav. 2002 Sep;77(1):167-76.
- ↑ Moncho-Bogani J, Martinez-Garcia F, Novejarque A, Lanuza E. Attraction to sexual pheromones and associated odorants in female mice involves activation of the reward system and basolateral amygdala. Eur J Neurosci. 2005 Apr;21(8):2186-98.
- ↑ "Researchers Prove A Single Memory Is Processed In Three Separate Parts Of The Brain" http://www.sciencedaily.com/releases/2006/02/060202182107.htm
- ↑ Ferry B, Roozendaal B, McGaugh J (1999). "Role of norepinephrine in mediating stress hormone regulation of long-term memory storage: a critical involvement of the amygdala". Biol Psychiatry. 46 (9): 1140–52. doi:10.1016/S0006-3223(99)00157-2. PMID 10560021.
- ↑ "Cultivating compassion: Neuroscientific and behavioral approaches" a talk given by Richard J. Davidson found online at "Archived copy". Archived from the original on 14 July 2010. Retrieved 2010-07-04.
- ↑ Lutz, Antoine; Brefczynski-Lewis, Julie; Johnstone, Tom; Davidson, Richard J. (2008). Baune, Bernhard, ed. "Regulation of the Neural Circuitry of Emotion by Compassion Meditation: Effects of Meditative Expertise". PLoS ONE. 3 (3): e1897. doi:10.1371/journal.pone.0001897. PMC 2267490
 . PMID 18365029.
. PMID 18365029. - ↑ Hutcherson, Cendri A.; Seppala, Emma M.; Gross, James J. (2008). "Loving-kindness meditation increases social connectedness". Emotion. 8 (5): 720–4. doi:10.1037/a0013237. PMID 18837623.
- ↑ Paré D.; Collins D.R.; Pelletier J.G. (2002). "Amygdala oscillations and the consolidation of emotional memories". Trends in Cognitive Sciences. 6 (7): 306–314. doi:10.1016/S1364-6613(02)01924-1. PMID 12110364.
- ↑ Asari T, Konishi S, Jimura K, Chikazoe J, Nakamura N, Miyashita Y (2010). "Amygdalar enlargement associated with unique perception". Cortex. 46 (1): 94–99. doi:10.1016/j.cortex.2008.08.001. PMID 18922517.
- ↑ Brown, S.; Shafer, E. (1888). "An investigation into the functions of the occipital and temporal lobes of the monkey's brain". Philosophical Transactions of the Royal Society B. 179: 303–327. doi:10.1098/rstb.1888.0011.
- ↑ Kluver, H.; Bucy, P. (1939). "Preliminary analysis of function of the temporal lobe in monkeys". Archives of Neurology. 42 (6): 979–1000. doi:10.1001/archneurpsyc.1939.02270240017001.
- ↑ Bucher, K.; Myersn, R.; Southwick, C. (1970). "Anterior temporal cortex and maternal behaviour in monkey". Neurology. 20 (4): 415. doi:10.1212/wnl.20.4.402. PMID 4998075.
- ↑ Aggleton, JP.; Passingham, RE. (1981). "Syndrome produced by lesions of the amygdala in monkeys (Macaca mulatta)". Journal of Comparative and Physiological Psychology. 95 (6): 961–977. doi:10.1037/h0077848. PMID 7320283.
- ↑ http://pn.psychiatryonline.org/content/40/9/37.full[][]
- ↑ Donegan, Nelson H; Sanislow, CA; Blumberg, HP; Fulbright, RK; Lacadie, C; Skudlarski, P; Gore, JC; Olson, IR; McGlashan, TH; et al. (2003). "Amygdala hyperreactivity in borderline personality disorder: implications for emotional dysregulation". Biological Psychiatry. 54 (11): 1284–1293. doi:10.1016/S0006-3223(03)00636-X. PMID 14643096.
- ↑ R. J. R. Blair (23 April 2008). "The amygdala and ventromedial prefrontal cortex: functional contributions and dysfunction in psychopathy". Philosophical Transactions of the Royal Society B: Biological Sciences. 363 (1503): 2557–2565. doi:10.1098/rstb.2008.0027. PMC 2606709
 . PMID 18434283.
. PMID 18434283. - ↑ Studying Brain Activity Could Aid Diagnosis Of Social Phobia. Monash University. 19 January 2006.
- ↑ Sheline; Barch, DM; Donnelly, JM; Ollinger, JM; Snyder, AZ; Mintun, MA; et al. (2001). "Increased amygdala response to masked emotional faces in depressed subjects resolves with antidepressant treatment: an fMRI study". Biological Psychiatry. 50 (9): 651–658. doi:10.1016/S0006-3223(01)01263-X. PMID 11704071.
- ↑ Blumberg; Kaufman, J; Martin, A; Whiteman, R; Zhang, JH; Gore, JC; Charney, DS; Krystal, JH; Peterson, BS; et al. (2003). "Amygdala and hippocampal volumes in adolescents and adults with bipolar disorder". Arch Gen Psychiatry. 60 (12): 1201–8. doi:10.1001/archpsyc.60.12.1201. PMID 14662552.
- ↑ Schultz RT (2005). "Developmental deficits in social perception in autism: the role of the amygdala and fusiform face area". Int J Dev Neurosci. 23 (2–3): 125–41. doi:10.1016/j.ijdevneu.2004.12.012. PMID 15749240.
- ↑ Williams, Leanne M.; Belinda J. Liddell; Andrew H. Kemp; Richard A. Bryant; Russell A. Meares; Anthony S. Peduto; Evian Gordon (2006). "Amygdala-prefrontal dissociation of subliminal and supraliminal fear". Human Brain Mapping. 27 (8): 652–661. doi:10.1002/hbm.20208. PMID 16281289.
- ↑ Brain Activity Reflects Complexity Of Responses To Other-race Faces, Science Daily, 14 December 2004
- ↑ Tsuchiya N, Moradi F, Felsen C, Yamazaki M, Adolphs R (2009). "Intact rapid detection of fearful faces in the absence of the amygdala". Nature Neuroscience. 12 (10): 1224–12225. doi:10.1038/nn.2380. PMC 2756300
 . PMID 19718036.
. PMID 19718036. - ↑ Vyas; Kim, SK; Giacomini, N; Boothroyd, JC; Sapolsky, RM; et al. (2007). "Behavioral changes induced by Toxoplasma infection of rodents are highly specific to aversion of cat odors". Proceedings of the National Academy of Sciences of the United States of America. 104 (15): 6442–7. doi:10.1073/pnas.0608310104. PMC 1851063
 . PMID 17404235.
. PMID 17404235. - ↑ Gazzaniga, M.S., Ivry, R.B., & Mangun, G.R. (2009). Cognitive neuroscience: the biology of the mind. NY: W.W.Norton&Company.
- ↑ Swaab, D. F. (2008). "Sexual orientation and its basis in brain structure and function". Proceedings of the National Academy of Sciences of the United States of America. 105 (30): 10273–4. doi:10.1073/pnas.0805542105. PMC 2492513
 . PMID 18653758.
. PMID 18653758. - ↑ Swaab, Dick F. (2007). "Sexual differentiation of the brain and behavior". Best Practice & Research Clinical Endocrinology & Metabolism. 21 (3): 431–44. doi:10.1016/j.beem.2007.04.003. PMID 17875490.
- ↑ Bickart, Kevin C; Wright, Christopher I; Dautoff, Rebecca J; Dickerson, Bradford C; Barrett, Lisa Feldman (2010). "Amygdala volume and social network size in humans". Nature Neuroscience. 14 (2): 163–4. doi:10.1038/nn.2724. PMC 3079404
 . PMID 21186358.
. PMID 21186358. - ↑ Szalavitz, Maia (28 December 2010). "How to Win Friends: Have a Big Amygdala?". Time. Retrieved 30 December 2010.
- ↑ Bzdok, D.; Langner, R.; Caspers, S.; Kurth, F.; Habel, U.; Zilles, K.; Laird, A.; Eickhoff, Simon B. (2010). "ALE meta-analysis on facial judgments of trustworthiness and attractiveness". Brain Structure and Function. 215 (3–4): 209–23. doi:10.1007/s00429-010-0287-4. PMID 20978908.
- ↑ Mormann, F.; Niediek, J.; Tudusciuc, O.; Quesada, C. M.; Coenen, V. A.; Elger, C. E.; Adolphs, R. (2015). "Neurons in the human amygdala encode face identity, but not gaze direction". Nature Neuroscience. 18 (11): 1568–1570. doi:10.1038/nn.4139.
- ↑ Huijgen, J.; Dinkelacker, V.; Lachat, F.; Yahia-Cherif, L.; El Karoui, I.; Lemaréchal, J.; George, N. (2015). "Amygdala processing of social cues from faces: An intracrebral EEG study". Social Cognitive And Affective Neuroscience. 10 (11): 1568–1576.
- ↑ Buchanan, T.W., Tranel, D. & Adolphs, R. in The Human Amygdala (eds. Whalen, P.J. & Phelps, E.A.) 289–318 (Guilford, New York, 2009).
- 1 2 Kennedy DP, Gläscher J, Tyszka JM, Adolphs R (2009). "Personal space regulation by the human amygdala". Nature Neuroscience. 12 (10): 1226–1227. doi:10.1038/nn.2381. PMC 2753689
 . PMID 19718035.
. PMID 19718035. - ↑ T.L. Brink. (2008) Psychology: A Student Friendly Approach. "Unit 4: The Nervous System." pp 61
- ↑ Feinstein, Justin S.; Adolphs, Ralph; Damasio, Antonio; Tranel, Daniel (2011). "The Human Amygdala and the Induction and Experience of Fear". Current Biology. 21 (1): 34–8. doi:10.1016/j.cub.2010.11.042. PMC 3030206
 . PMID 21167712.
. PMID 21167712. - ↑ Staut, C. C. V.; Naidich, T. P. (1998). "Urbach-Wiethe Disease(Lipoid Proteinosis)". Pediatric Neurosurgery. 28 (4): 212–214. doi:10.1159/000028653. PMID 9732251.
- ↑ http://bps-research-digest.blogspot.com/2013/02/extreme-fear-experienced-without.html[]
- ↑ Stephens, D. N; Duka, T. (2008). "Cognitive and emotional consequences of binge drinking: Role of amygdala and prefrontal cortex". Philosophical Transactions of the Royal Society B. 363 (1507): 3169–79. doi:10.1098/rstb.2008.0097. PMC 2607328
 . PMID 18640918.
. PMID 18640918. - ↑ Marinkovic, Ksenija; Oscar-Berman, Marlene; Urban, Trinity; o’Reilly, Cara E.; Howard, Julie A.; Sawyer, Kayle; Harris, Gordon J. (2009). "Alcoholism and Dampened Temporal Limbic Activation to Emotional Faces". Alcoholism: Clinical and Experimental Research. 33 (11): 1880–92. doi:10.1111/j.1530-0277.2009.01026.x. PMC 3543694
 . PMID 19673745.
. PMID 19673745. - ↑ Newton, P; Ron, D (2007). "Protein kinase C and alcohol addiction". Pharmacological Research. 55 (6): 570–7. doi:10.1016/j.phrs.2007.04.008. PMID 17566760.
- ↑ Lesscher, H. M. B.; Wallace, M. J.; Zeng, L.; Wang, V.; Deitchman, J. K.; McMahon, T.; Messing, R. O.; Newton, P. M. (2009). "Amygdala protein kinase C epsilon controls alcohol consumption". Genes, Brain and Behavior. 8 (5): 493–9. doi:10.1111/j.1601-183X.2009.00485.x. PMC 2714877
 . PMID 19243450.
. PMID 19243450. - ↑ Ziabreva, Irina; Poeggel, Gerd; Schnabel, Reinhild; Braun, Katharina (2003). "Separation-induced receptor changes in the hippocampus and amygdala of Octodon degus: Influence of maternal vocalizations". The Journal of Neuroscience. 23 (12): 5329–36. PMID 12832558.
- ↑ Davis, M (1992). "The role of the amygdala in fear and anxiety". Annual Review of Neuroscience. 15: 353–375. doi:10.1146/annurev.ne.15.030192.002033. PMID 1575447.
- ↑ Carlson, Neil R. (12 January 2012). Physiology of Behavior. Pearson. p. 608. ISBN 978-0205239399.
- ↑ Laura A; Thomas; et al. (2013). "Elevated amygdala responses to emotional faces in youths with chronic irritability or bipolar disorder.". Neuroimage Clinical. 2 (2): 637–645. doi:10.1016/j.nicl.2013.04.007. PMC 3746996
 . PMID 23977455.
. PMID 23977455. - ↑ M. T. Keener; et al. (2012). "Dissociable patterns of medial prefrontal and amygdala activity to face identity versus emotion in bipolar disorder.". Psychological Medicine. 42 (9): 1913–1924. doi:10.1017/S0033291711002935. PMC 3685204
 . PMID 22273442.
. PMID 22273442. - ↑ http://www.cell.com/current-biology/abstract/S0960-9822%2811%2900289-2
External links
| Look up amygdala in Wiktionary, the free dictionary. |
 Media related to amygdala at Wikimedia Commons
Media related to amygdala at Wikimedia Commons- Stained brain slice images which include the "amygdala " at the BrainMaps project
- international committee for amygdala and health studies

