Antimony pentafluoride
| |||
| Names | |||
|---|---|---|---|
| IUPAC name
antimony(V) fluoride | |||
| Other names
antimony pentafluoride pentafluoridoantimony | |||
| Identifiers | |||
| 7783-70-2 | |||
| 3D model (Jmol) | Interactive image | ||
| ChemSpider | 22963 | ||
| ECHA InfoCard | 100.029.110 | ||
| PubChem | 24557 | ||
| RTECS number | CC5800000 | ||
| UN number | 1732 | ||
| |||
| |||
| Properties | |||
| SbF5 | |||
| Molar mass | 216.74 g/mol | ||
| Appearance | colorless oily liquid hygroscopic | ||
| Odor | pungent | ||
| Density | 2.99 g/cm3 [1] | ||
| Melting point | 8.3 °C (46.9 °F; 281.4 K) | ||
| Boiling point | 149.5 °C (301.1 °F; 422.6 K) | ||
| Reacts | |||
| Solubility | soluble in KF, liquid SO2 | ||
| Hazards | |||
| Safety data sheet | ICSC 0220 | ||
| EU classification (DSD) |
Harmful (Xn) Dangerous for the environment (N) | ||
| R-phrases | R20/22, R51/53 | ||
| S-phrases | (S2), S61 | ||
| NFPA 704 | |||
| US health exposure limits (NIOSH): | |||
| PEL (Permissible) |
TWA 0.5 mg/m3 (as Sb)[2] | ||
| REL (Recommended) |
TWA 0.5 mg/m3 (as Sb)[2] | ||
| Related compounds | |||
| Other anions |
Antimony pentachloride | ||
| Other cations |
Phosphorus pentafluoride Arsenic pentafluoride Bismuth pentafluoride | ||
| Related compounds |
Antimony trifluoride | ||
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| | |||
| Infobox references | |||
Antimony pentafluoride is the inorganic compound with the formula SbF5. This colourless, viscous liquid is a valuable Lewis acid and a component of the superacid fluoroantimonic acid, the strongest known acid. It is notable for its Lewis acidity and its ability to react with almost all known compounds.[3]
Preparation
Antimony pentafluoride is prepared by the reaction of antimony pentachloride with anhydrous hydrogen fluoride:[4]
- SbCl5 + 5 HF → SbF5 + 5 HCl
It can also be prepared from antimony trifluoride and fluorine.[5]
Structure and chemical reactions
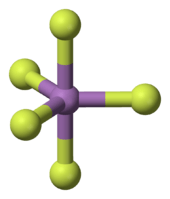
In the gas phase, SbF5 adopts a trigonal bipyramidal structure of D3h point group symmetry (see picture). The material adopts a more complicated structure in the liquid and solid states. The liquid contains polymers wherein each Sb is octahedral, the structure being described with the formula [SbF4(μ-F)2]n ((μ-F) denotes the fact that fluoride centres bridge two Sb centres). The crystalline material is a tetramer, meaning that it has the formula [SbF4(μ-F)]4. The Sb-F bonds are 2.02 Å within the eight-membered Sb4F4 ring; the remaining fluoride ligands radiating from the four Sb centers are shorter at 1.82 Å.[6] The related species PF5 and AsF5 are monomeric in the solid and liquid states, probably due to the smaller sizes of the central atom, which limits their coordination number. BiF5 is a polymer.[7]
In the same way that SbF5 enhances the Brønsted acidity of HF, it augments the oxidizing power of F2. This effect is illustrated by the oxidation of oxygen:[8]
- 2 SbF5 + F2 + 2 O2 → 2 [O
2]+
[SbF
6]−
Antimony pentafluoride has also been used in the first discovered chemical reaction that produces fluorine gas from fluoride compounds:
- 4 SbF
5 + 2 K
2MnF
6 → 4 KSbF
6 + 2 MnF
3 + F
2
The driving force for this reaction is the high affinity of SbF5 for F−
, which is the same property that recommends the use of SbF5 to generate superacids.
Hexafluoroantimonate
SbF5 is a strong Lewis acid, exceptionally so toward sources of F− to give the very stable anion [SbF6]−, called hexafluoroantimonate. [SbF6]− is a weakly coordinating anion akin to PF6−. Although it is only weakly basic, [SbF6]− does react with additional SbF5 to give a centrosymmetric adduct:
- SbF5 + [SbF6]− → [Sb2F11]−
Safety
SbF5 reacts violently with many compounds, often releasing dangerous hydrogen fluoride. It is corrosive to the skin and eyes.[9][10]
References
- ↑ Lide, David R., ed. (2006). CRC Handbook of Chemistry and Physics (87th ed.). Boca Raton, FL: CRC Press. ISBN 0-8493-0487-3.
- 1 2 "NIOSH Pocket Guide to Chemical Hazards #0036". National Institute for Occupational Safety and Health (NIOSH).
- ↑ Olah, G. A.; Prakash, G. K. S.; Wang, Q.; Li, X.-y."Antimony(V) Fluoride" in Encyclopedia of Reagents for Organic Synthesis (Ed: L. Paquette) 2004, J. Wiley & Sons, New York. doi:10.1002/047084289.
- ↑ Sabina C. Grund, Kunibert Hanusch, Hans J. Breunig, Hans Uwe Wolf "Antimony and Antimony Compounds" in Ullmann's Encyclopedia of Industrial Chemistry 2006, Wiley-VCH, Weinheim. doi: 10.1002/14356007.a03_055.pub2
- ↑ Handbook of Preparative Inorganic Chemistry, 2nd Ed. Edited by G. Brauer, Academic Press, 1963, NY. Vol. 1. p. 200.
- ↑ Edwards, A. J.; Taylor, P. "Crystal structure of Antimony Pentafluoride" Journal of the Chemical Society, Chemical Communications 1971, pp. 1376-7.doi:10.1039/C29710001376
- ↑ Holleman, A. F.; Wiberg, E. "Inorganic Chemistry" Academic Press: San Diego, 2001. ISBN 0-12-352651-5.
- ↑ Shamir, J.; Binenboym, J. "Dioxygenyl Salts" Inorganic Syntheses 1973, XIV, 109-122. ISSN 0073-8077
- ↑ International Programme on Chemical Safety (2005). "Antimony pentafluoride". Commission of the European Communities (CEC). Retrieved 2010-05-10.
- ↑ Barbalace, Kenneth (2006). "Chemical Database - Antimony Pentafluoride". Environmental Chemistry. Retrieved 2010-05-10.
External links
- WebBook page for SbF5
- National Pollutant Inventory - Antimony and compounds fact sheet
- National Pollutant Inventory - Fluoride compounds fact sheet