Asymmetric addition of alkynylzinc compounds to aldehydes
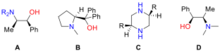
Asymmetric addition of alkynylzinc compounds to aldehydes is an enantioselective chemical reaction where alkynylzinc reagents react with aldehydes to generate propargyl alcohols.[1] Propargyl alcohols are versatile precursors to various complex molecules making the asymmetric addition of alkynylzinc compounds to aldehydes a particularly useful method for the synthesis of natural products and pharmaceutical drugs. For example, Carreira and co-workers have used this asymmetric reaction for the synthesis of natural product Leucascandrolide A, one of the first powerfully bioactive metabolites isolated from a calcareous sponge.[2] Various chiral ligands have been developed for the asymmetric addition of alkynylzinc compounds to aldehydes. Because of the acidity of the terminal alkynyl proton, the alkynylzinc compounds can be generated in situ from reaction of terminal alkynes with alkylzincs or Zn(OTf)2.
The first example of catalytic asymmetric addition of alkynylzinc compounds to aldehydes was reported by Kenso Soai and co-workers in 1990.[3] In their experiments, chiral amino alcohols and amines (A to C in Figure "Examples of ligands used in asymmetric addition of alkynylzinc compounds to aldehydes") were used as ligands, and the alkynylzinc reagent was prepared from reaction of alkyne with diethylzinc. Although the yields were high, but the highest enantiomeric excess achieved was only 34% with 5 mol% ligand loading. Erick Carreira and co-workers reported high enantiomeric excess using a chiral amino alcohol, N-methylephedrine (D).[4] Stoichiometric amount of D was used in their reactions, and up to 99% of enantiomeric excess was achieved at room temperature for a broad range of aldehydes. In Carreira’s experiments, the alkynylzinc reagent was generated from the reaction of alkyne with Zn(OTf)2 in the presence of an amine base.