Atrane
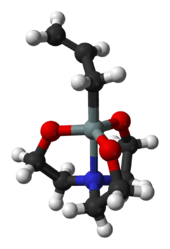
An Atrane is a tricyclic molecule with three five-membered rings. It has a transannular dative bond by a nitrogen atom, depicted as A.[1]
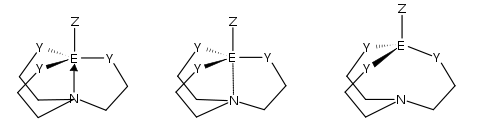
Various atranes are named depending on the central element, e.g. "silatrane" (E = silicon); "boratrane" (E = boron); "phosphatrane" (E = phosphorus), etc. It is also proposed that when Y = nitrogen, the prefix "aza" be inserted before element + "atrane" (azasilatrane, for example) because atranes wherein E = silicon and Y = oxygen have been referred to as just "silatranes".[2]
Silatranes exhibit unusual properties as well as biological activity in which the coordination of nitrogen to silane plays an important role. Some derivatives such as phenylsilatrane are highly toxic.
The transannular coordinate bond in atranes can be stretched (quasiatranes, B) and even broken (proatranes, C) by controlling their stereoelectronic properties. Proazaphosphatrane is a very strong non-ionic base and is utilized in various types of organic synthesis as an efficient catalyst.
The strength of the transannular interaction depends on the electronegativity of the participating atoms and the size of the rings.
The name "atrane" was first proposed by Mikhail Grigorievich Voronkov (Воронков, Михаил Григорьевич).[1]
See also
References
- 1 2 Voronkov, Mikhail G.; Baryshok, Viktor P. "Atranes - a new generation of biologically active substances" (in Russian) Vestnik Rossiiskoi Akademii Nauk 2010, volume 80, 985-992.
- ↑ Verkade, John G. (1994). "Main group atranes: Chemical and structural features". Coordination Chemistry Reviews. 137: 233. doi:10.1016/0010-8545(94)03007-D.
| Wikimedia Commons has media related to atranes. |