Bromopentaamminecobalt(III) bromide
 | |
| Identifiers | |
|---|---|
| 14283-12-6 | |
| Properties | |
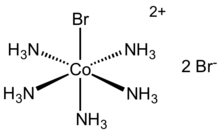
| [Co(NH3)5Br]Br2 | |
| Molar mass | 383.798 g/mol |
| Appearance | Purple |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Bromopentaamminecobalt(III) bromide is a cobalt coordination compound with the formula [Co(NH3)5Br]Br2. It is a purple, water-soluble solid.
Synthesis and reactions
The title compound is prepared by oxidation of a solution of cobalt(II) salts in aqueous ammonia.[1]
- 2 CoBr2 + 8 NH3 + 2 NH4Br + H2O2 → 2 [Co(NH3)5Br]Br2 + 2 H2O
It was first reported in the 1870s, before the structure or even formulae were understood for such complexes. This early work showed that only two thirds of the bromide groups were exchangeable with other anions such as nitrate and dithionate.[2]
The complex undergoes aquation, meaning that bromide is displaced by water:
- [Co(NH3)5Br]Br2 + H2O → [Co(NH3)5(H2O)]Br3
This process is catalyzed by platinum.[3]
References
- ↑ Diehl, Harvey; Clark, Helen; Willard, H. H. (1939). "Bromopentamminocobalti Bromide". Inorganic Syntheses. 1: 186. doi:10.1002/9780470132326.ch66.
- ↑ Jörgensen, S. M. (1879). "Beiträge zur Chemie der Kobaltammoniakverbindungen. II. Ueber die Bromopurpureokobaltsalze". Journal für Praktische Chemie. 19: 49. doi:10.1002/prac.18790190110.
- ↑ Archer, M. D.; Spiro, M. (1970). "Heterogeneous Catalysis in Solution. Part VIII. Catalysis of the Aquation of the Bromopentaamminecobalt(III) Ion by Metallic Platinum". Journal of the Chemical Society (1): 78–81.
Further reading
- Loehlin, James H. "The Study of a Cobalt Complex-A Laboratory Project." Journal of Chemical Education. ACS Publications, Dec. 1982. Web.
- Williams, Gregory M.; Olmsted, John; Brekda, Andrew P, December 1989.“Coordination Complexes of Cobalt.” Journal of Chemical Education 66 number 12.
This article is issued from Wikipedia - version of the 6/8/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.