Catecholborane
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Catecholborane | |
| Other names
1,3,2-benzodioxaborole 7,9-dioxa-8λ2-borabicyclo[4.3.0]nona-1,3,5-triene | |
| Identifiers | |
| 274-07-7 | |
| 3D model (Jmol) | Interactive image Interactive image |
| ChemSpider | 10617125 |
| ECHA InfoCard | 100.005.447 |
| |
| |
| Properties | |
| C6H5BO2 | |
| Molar mass | 119.92 g/mol |
| Appearance | Colorless liquid |
| Density | 1.125 g/cm3, liquid |
| Melting point | 12 °C (54 °F; 285 K) |
| Boiling point | 50 °C (122 °F; 323 K) at 50 mmHg |
| Hazards | |
| R-phrases | R11, R14, R34 |
| S-phrases | S16, S26, S36/37/39, S43, S45 |
| NFPA 704 | |
| Flash point | 2 °C (36 °F; 275 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Catecholborane (abbreviated HBcat) is an organoboron compound that is useful in organic synthesis. This colourless liquid is a derivative of catechol and a borane, having the formula C6H4O2BH.
Synthesis and structure
Traditionally catecholborane is produced by treating catechol with borane (BH3) in a cooled solution of THF. However, this method results in a loss of 2 mole equivalents of the hydride. Nöth and Männig devised a more economical method involves the reaction of alkali-metal boron hydride (LiBH4, NaBH4, of KBH4) with tris(catecholato)bisborane in an ethereal solvent such as diethyl ether.[1] In 2001 Herbert Brown released an additional procedure for catecholborane synthesis. His method involves treating tri-O-phenylene bis-borate with diborane in a solution of either triglyme or tetraglyme. Brown claimed his method produces 85% yield of 97% pure product, catecholborane.[2]
Unlike borane itself or alkylboranes, catechol borane exists as a monomer. This behavior is a consequence of the electronic influence of the alkoxy groups that diminish the Lewis acidity of the boron centre. Pinacolborane adopts a similar structure.
Reactions
Catechol borane is less reactive than borane itself.
Preparation of an organoborane
When catecholborane is treated with an alkyne, usually a terminal alkyne, through hydroboration a trans vinylborane is formed. The product is a precursor to the Suzuki reaction.[3]

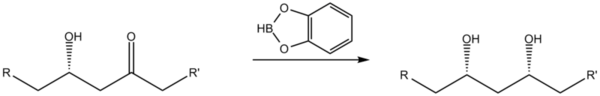
Reduction of β-hydroxy ketones
Catecholborane may be used as a stereoselective reducing agent when converting β-hydroxy ketones to syn 1,3-diols.[5]
References
- ↑ Process for producing catecholborane - Patent 4739096
- ↑ New Economical, Convenient Procedures for the Synthesis of Catecholborane
- ↑ Janice Gorzynski Smith. Organic Chemistry: Second Ed. 2008. pp 1007
- ↑ Norio Miyaura (1990). "Discussion Addendum for:PALLADIUM-CATALYZED REACTION OF 1-ALKENYLBORONATES WITH VINYLIC HALIDES: (1Z,3E)-1-PHENYL-1,3-OCTADIENE". Org. Synth.; Coll. Vol., 68, p. 130
- ↑ http://evans.harvard.edu/pdf/evans135.pdf
