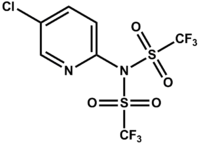
Comins' reagent
 | |
 | |
| Identifiers | |
|---|---|
| 145100-51-2 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 344376 |
| ECHA InfoCard | 100.157.321 |
| PubChem | 388544 |
| |
| |
| Properties | |
| C7H3ClF6N2O4S2 | |
| Molar mass | 392.67 g·mol−1 |
| Appearance | White solid |
| Melting point | 45 °C (113 °F; 318 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
The Comins' reagent is a triflyl compound that is used to synthesize vinyl triflates from the corresponding ketone enolates or dienolates.[1]

It was first reported in 1992 by Daniel Comins from North Carolina State University.[2] The vinyl triflates prepared are useful as substrates in the Suzuki reaction.[3]
References
- ↑ Mundy, Bradford P.; Ellerd, Michael G.; Favaloro, Frank G., Jr. (2005). Name Reactions and Reagents in Organic Synthesis (2nd ed.). ISBN 0471228540.
- ↑ Comins, Daniel L.; Dehghani, Ali (1992). "Pyridine-Derived Triflating Reagents: An Improved Preparation of Vinyl Triflates from Metallo Enolates". Tetrahedron Letters. 33 (42): 6299–6302. doi:10.1016/S0040-4039(00)60957-7.
- ↑ Miyaura, Norio; Suzuki, Akira (1995). "Palladium-Catalyzed Cross-Coupling Reactions of Organoboron Compounds". Chemical Reviews. 95: 2457–2483. doi:10.1021/cr00039a007.
This article is issued from Wikipedia - version of the 12/3/2015. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.