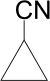
Cyclopropyl cyanide
 | |
| Names | |
|---|---|
| IUPAC name
cyclopropanecarbonitrile | |
| Other names
Cyclopropanecarbonitrile,Cyanocyclopropane, | |
| Identifiers | |
| 5500-21-0 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 71940 |
| ECHA InfoCard | 100.024.397 |
| PubChem | 79637 |
| |
| Properties | |
| C4H5N | |
| Molar mass | 67.0892g/mol[1] |
| Appearance | clear to light yellow liquid |
| Density | 0.911g/mL |
| Melting point | −25 °C (−13 °F; 248 K) |
| Boiling point | 135 °C (275 °F; 408 K) |
| soluble in water[2] | |
| log P | 1.196 |
| Hazards | |
| Main hazards | Toxic, hazardous if inhaled, contacted with skin, or swallowed |
| Flash point | 40 °C (104 °F; 313 K) |
| not applicable | |
| Thermochemistry | |
| Std enthalpy of combustion (ΔcH |
182.7 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Cyclopropyl cyanide is the smallest carbon ring molecule with a cyanide group attached to it. It is mainly known by its involvement in experiments with microwave spectroscopy, rotational spectroscopy and photodissociation. In 1958, cyclopropyl cyanide was first studied for its rotational spectra, by Friend and Dailey. Additional experiment involving cyclopropyl cyanide was the determination of the molecule's dipole moment through spectroscopy experiments, by Carvalho in 1967.[3]
Production
One method of synthesis of cyclopropyl cyanide is through reducing methy 3-cyanopropionate with lithium borodeuteride. This reduction yields an alcohol, which is further reacted triphenylphosphine and carbon tetrachloride. The product, 4-chlorobutyronitrile, is reacted with sodium iodide to replace the chloride with the iodide. Lastly, the molecule is turned into a cyclic molecule by reacting with sodium hydride.[4] Another proposed method of synthesis involves reacting 4-chlorobutyronitrile with sodium amide. The sodium amide is produced by reacting solid sodium and liquid ammonia.[5]
Reactions
Cyclopropyl cyanide, when heated to 660-760K and under pressure of 2-89torr, becomes cis and trans crotonitrile and allyl cyanide molecules, with some presence of methacrylonitrile. This is an isomerization reaction that is homogeneous with rate of first order. The reaction result is due to the biradical mechanism, involving the formation of carbon radicals as the three carbon ring opens up. The radicals then react to yield carbon carbon double bonds.[6]
References
- ↑ "cyclopropanecarbonitrile - Compound Summary". PubChem.
- ↑ "Cyclopropyl cyanide". Chemical Book.
- ↑ Bizzocchi, Luca; Claudio Degli Esposti; Luca Dore; Zbigniew Kisiel (September–October 2008). "Submillimetre-wave spectrum, 14N-hyperfine structure, and dipole moment of cyclopropyl cyanide". Journal of Molecular Spectroscopy. 251 (1-2): 138–144. doi:10.1016/j.jms.2008.02.009.
- ↑ Edwards, William; David Glenn (11 September 1975). "SYNTHESIS OF CYCLOPROPYL CYANIDE-2,2-D,". JourmL of Labelled Compounds and Radiopharmaceuticals. 12 (1): 145–151. doi:10.1002/jlcr.2580120117.
- ↑ Schlatter (1955). Organic Syntheses. 3: 223. Missing or empty
|title=(help) - ↑ LUCKRAFT; ROBINSON (1973). INTERNATIONAL JOURNAL OF CHEMICAL KINETICS. 5: 137–147. doi:10.1002/kin.550050112. Missing or empty
|title=(help)