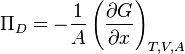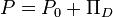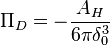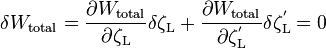Disjoining pressure
Disjoining pressure (symbol Πd), in surface chemistry, according to an IUPAC definition,[1] arises from an attractive interaction between two surfaces. For two flat and parallel surfaces, the value of the disjoining pressure (i.e., the force per unit area) can be calculated as the derivative of the Gibbs energy of interaction per unit area in respect to distance (in the direction normal to that of the interacting surfaces). There is also a related concept of disjoining force, which can be viewed as disjoining pressure times the surface area of the interacting surfaces.
The concept of disjoining pressure was introduced by Derjaguin (1936) as the difference between the pressure in a region of a phase adjacent to a surface confining it, and the pressure in the bulk of this phase.[2][3]
Description
Disjoining pressure can be expressed as:[4]
where:
- Πd - disjoining pressure, N/m2
- A - the surface area of the interacting surfaces, m2
- G - total Gibbs energy of the interaction of the two surfaces, J
- x - distance, m
- indices T, V and A signify that the temperature, volume, and the surface area remain constant in the derivative.
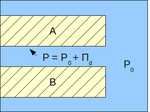
Using the concept of the disjoining pressure, the pressure in a film can be viewed as:[4]
where:
- P - pressure in a film, Pa
- P0 - pressure in the bulk of the same phase as that of the film, Pa.
Disjoining pressure is interpreted as a sum of several interactions: dispersion forces, electrostatic forces between charged surfaces, interactions due to layers of neutral molecules adsorbed on the two surfaces, and the structural effects of the solvent.
Classic theory predicts that the disjoining pressure of a thin liquid film on a flat surface as follows,[5]
where:
- AH - Hamaker constant, J
- δ0 - liquid film thickness, m
For a solid-liquid-vapor system where the solid surface is structured, the disjoining pressure is affected by the solid surface profile, ζS , and the meniscus shape, ζL [6]
where:
- ω(ρ,z) - solid-liquid potential, J/m6
The meniscus shape can be by minimization of total system free energy as follows [7]
where:
- Wtotal - , total system free energy including surface excess energy and free energy due to solid-liquid interactions, J/m2
- ζL - meniscus shape, m
- ζ'L - slope of meniscus shape, 1
See also
References
- ↑ "Disjoining pressure". Entry in the IUPAC Compendium of Chemical Terminology ("The Gold Book"), the International Union of Pure and Applied Chemistry, 2nd edition, 1997
- ↑ See:
- Дерягин, Б. В. and Кусаков М. М. (Derjaguin, B. V. and Kusakov, M. M.) (1936) "Свойства тонких слоев жидкостей" (The properties of thin layers of liquids), Известия Академии Наук СССР, Серия Химическая (Proceedings of the Academy of Sciences of the USSR, Chemistry series), 5 : 741-753.
- Derjaguin, B. with E. Obuchov (1936) "Anomalien dünner Flussigkeitsschichten. III. Ultramikrometrische Untersuchungen der Solvathüllen und des "elementaren" Quellungsaktes" (Anomalies of thin liquid layers. III. Investigations via ultramicroscope measurements of solvation shells and of the "elementary" act of imbibition), Acta Physicochimica U.R.S.S., 5 : 1-22.
- ↑ A. Adamson, A. Gast, "Physical Chemistry of Surfaces", 6th edition, John Wiley and Sons Inc., 1997, page 247.
- 1 2 Hans-Jürgen Butt, Karlheinz Graf, Michael Kappl,"Physics and chemistry of interfaces", John Wiley & Sons Canada, Ltd., 1 edition, 2003, page 95 (Google books)
- ↑ Jacob N. Israelachvili,"Intermolecular and Surface Forces", Academic Press, Revised Third edition, 2011, page 267-268 (Google books)
- ↑ Mark O. Robbins, David, Andelman, and Jean-François Joanny, Phys. Rev. A 43, 4344, (1991)
- ↑ Han Hu, Christopher R. Weinberger, and Ying Sun Nano Lett., 14 (12), pp 7131–7137 (2014)