Element collecting
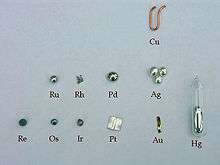

Element collecting is the hobby of collecting the chemical elements. Many element collectors simply enjoy finding peculiar uses of chemical elements. Others enjoy studying the properties of the elements, possibly engaging in amateur chemistry, and some simply collect elements for no practical reason. Some element collectors invest in elements, while some amateur chemists have amassed a large collection of elements—Oliver Sacks, for example.[1] In recent years, the hobby has gained popularity with media attention brought by element collectors like Theodore Gray.
Acquiring elements
Some collectors attempt to collect very high purity samples of each element. Others prefer to find the element in everyday use. Some are averse to collecting the element as a compound or alloy, while others find this acceptable. Collectors may isolate elements in their own homes. Hydrogen, for example, can be easily isolated via the electrolysis of water.[2]
Some commercial retailers now cater to the element collecting community, even selling large quantities in sets,[3] since purchasing elements from large chemical companies like Sigma-Aldrich is frequently prohibited or uneconomical for individuals. Element collecting presents many challenges: some elements, such as mercury, are toxic. Others are extremely rare in commercial use, such as scandium. Some, such as caesium, are too reactive; others, such as gallium, react corrosively and very fast with aluminium (e.g., as structural material in aircraft).[4] Others, like radon, are radioactive and have half lives too short for practical collection to say nothing of their radioactive hazards: usually, only the stable elements from hydrogen to bismuth (except the radioactive technetium and promethium) are collected, with the exceptions of the extremely long-lived thorium and uranium. (Additionally, unsuccessful attempts have occasionally been made by private individuals to collect technetium, because it has a long half-life of several million years, is not used in nuclear weapons like the longer-lived plutonium, and decays to clean stability.)
See also
References
- ↑ Sacks, Oliver (2001). Uncle Tungsten: Memories of a Chemical Boyhood. Vintage Books. ISBN 0-375-40448-1.
- ↑ Gray, Theodore. "The Wooden Periodic Table Table". Retrieved 20 November 2010.
- ↑ Gray, Theodore. "How to Get Your Own Element Collection". Retrieved 8 March 2015.
- ↑ "Publication 52, Hazardous, Restricted, and Perishable Mail" (PDF). USPS. January 2008.