Ferrier rearrangement
The Ferrier rearrangement is an organic reaction that involves a nucleophilic substitution reaction combined with an allylic shift in a glycal (a 2,3-unsaturated glycoside). It was discovered by the carbohydrate chemist Robert J. Ferrier.[1][2]
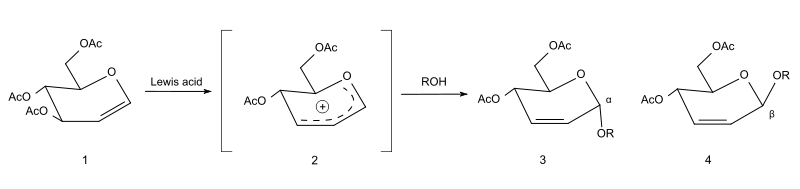
Mechanism
In the first step, a delocalized allyloxocarbenium ion (2) is formed, typically with the aid of a Lewis acid like indium(III) chloride or boron trifluoride. This ion reacts in situ with an alcohol, yielding a mixture of the α (3) and β (4) anomers of the 2-glycoside, with the double bond shifted to position 3,4.[3]
Examples
| Lewis acid | Alcohol | Conditions | Results |
|---|---|---|---|
| InCl3 | methanol | in dichloromethane | α:β = 7:1[4] |
| dioxane | water | heating | 75% yield[5] |
| SnCl4 | methanol | in dichloromethane, –78 °C, 10 min | 83% yield, α:β = 86:14[6] |
| BF3·O(C2H5)2 | isopropanol | in dichloromethane, RT, 24 hr | 95% yield[7][8] |
| ZnCl2 | ethanol | in toluene, RT, 30–60 min | 65–95% yield, α:β = 89:11[9][10] |
| BF3·O(C2H5)2 | benzyl alcohol | in dichloromethane, –20 °C to RT, 1 hr | 98% yield[11] |
Modifications
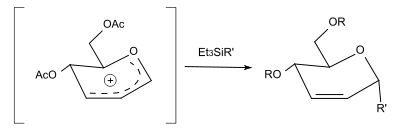
Forming of C-glycosides
By replacing the alcohol with a silane, C-glycosides can be formed. With triethylsilane (R'=H), the reaction yields a 2,3-unsaturated deoxy sugar.[3]
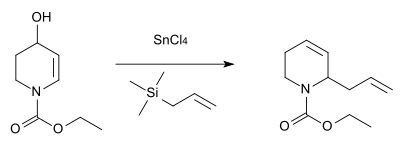
Nitrogen analogue
An analogous reaction with nitrogen as the heteroatom was described in 1984 for the synthesis of the antibiotic substance streptazolin.[12]
References
- ↑ Ferrier, Robert J. (1979). "Unsaturated Carbohydrates. Part 21. A Carboxylic Ring Closure of a Hex-5-enopyranoside Derivative". J. Chem. Soc., Perkin Trans. 1: 1455–1458. doi:10.1039/P19790001455.
- ↑ Ferrier, Robert J.; Zubkov, O. A. (2003). "Transformation of Glycals into 2,3-Unsaturated Glycosyl Derivatives". Org. React. 62. doi:10.1002/0471264180.or062.04. ISBN 0-471-26418-0.
- 1 2 Konstantinović, Stanimir; et al. (2001). "The Ferrier rearrangement as the key step in the synthesis of C7–C16-alkyl 2,3-dideoxy glucosides from glucose and C7–C16-alkanols" (PDF). J.Serb.Chem.Soc. 66 (8): 499–505.
- ↑ Boga, S. B.; Balasubramanian, K. K. (2004). "Indium trichloride catalyzed Ferrier rearrangement – facile synthesis of 2,3-unsaturated glycosides". Arkivoc: 87–102. (open access publication)
- ↑ Bert. Fraser- Reid; Bruno. Radatus (1970). "4,6-Di-O-acetyl-aldehydo-2,3-dideoxy-D-erythro-trans-hex-2-enose. Probable reason for the 'al' in Emil Fischer's triacetyl glucal". J. Am. Chem. Soc. 92 (17): 5288–5290. doi:10.1021/ja00720a087.
- ↑ Eleuterio Alvarez; Maria T. Diaz; Ricardo Perez; Jose L. Ravelo; Alicia Regueiro; Jose A. Vera; Dacil Zurita; Julio D. Martin (1994). "Simple Designs for the Construction of Complex trans-Fused Polyether Toxin Frameworks. A Linear Strategy Based on Entropically Favored Oxirane Ring Enlargement in Epoxycycloalkenes Followed by Carbon-Carbon or Carbon-Oxygen Bond-Forming Cyclizations". J. Org. Chem. 59 (10): 2848. doi:10.1021/jo00089a034.
- ↑ Ferrier, R. J.; Prasad, N. (1969). "Unsaturated carbohydrates. Part IX. Synthesis of 2,3-dideoxy-α-D-erythro-hex-2-enopyranosides from tri-O-acetyl-D-glucal". Journal of the Chemical Society C Organic (4): 570. doi:10.1039/J39690000570.
- ↑ Ferrier, R. J.; Prasad, N. (1969). "Unsaturated carbohydrates. Part X. Epoxidations and hydroxylations of 2,3-dideoxy-α-D-hex-2-enopyranosides. The four methyl 4,6-di-O-acetyl-2,3-anhydro-α-D-hexopyranosides". Journal of the Chemical Society C Organic (4): 575. doi:10.1039/J39690000575.
- ↑ Kelly, David R.; Picton, Mark R. (2000). "Catalytic tin radical mediated tricyclisations. Part 1. Monocyclisation studies". Journal of the Chemical Society Perkin Transactions 1 (10): 1559. doi:10.1039/b000661k.
- ↑ Kelly, David R.; Picton, Mark R. (2000). "Catalytic tin radical mediated tricyclisations. Part 2.". Journal of the Chemical Society Perkin Transactions 1 (10): 1571. doi:10.1039/b000662i.
- ↑ Donohoe, Timothy J.; Blades, Kevin; Helliwell, Madeleine (1999). "Synthesis of amino-sugars using the directed dihydroxylation reaction". Chemical Communications (17): 1733. doi:10.1039/a904991f.
- ↑ Kozikowski, AP, Pyeong-uk Park (1984). "Synthesis of 2-substituted .DELTA.3-piperidines: the nitrogen analog of the Ferrier rearrangement. An approach to streptazolin". J. Org. Chem. 49 (9): 1674–1676. doi:10.1021/jo00183a044.