FlAsH-EDT2
 | |
| Names | |
|---|---|
| Other names
Fluorescein Arsenical Hairpin Binder | |
| Identifiers | |
| 212118-77-9 | |
| 3D model (Jmol) | Interactive image |
| |
| Properties | |
| C24H18As2O5S4 | |
| Molar mass | 664.49 g·mol−1 |
| Appearance | Solid |
| Melting point | 169 to 172 °C (336 to 342 °F; 442 to 445 K) |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
FlAsH-EDT2 is an organoarsenic compound used in bioanalytical research.[1] It is a fluorescent label. The compound is a derivative of fluorescein. The fluorescin arsenical hairpin binder-ethanedithiol, more commonly known as FlAsH-EDT2, is a pale yellow or pinkish fluorogenic solid with the molecular formula (C2H4AsS2)2-(C20H10O5). It is used in site-specific labeling of living cells. It binds to proteins containing the tetracysteine motif Cys-Cys-Xxx-Xxx-Cys-Cys and becomes fluorescent. It has been seen in studies however that it will also bind non-specifically, meaning simply a site other than the main site of interest (CCXXCC), to endogenous cysteine-rich proteins.
Preparation
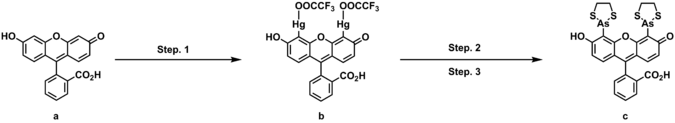
FlAsH-EDT2 can be prepared in three steps from fluorescein. In the first step, fluorescein is treated with mercury oxide and tetrafluoroacetic acid to produce fluorescein mercuric acetate, a yellow orange solid upon the completion of purification. Treating fluorescein mercuric acetate with arsenic trichloride and palladium acetate under the inert atmosphere goes through the transmetallation to give a yellow solution. To this reaction mixture, addition of 1,2-ethanedithiol functionalize thiarsolanyl to achieve the desired product, FlAsH-EDT2(a pale yellow or pinkish solid).[2]
Formation of FlAsH-TC adduct
Many studies show that trivalent arsenic compounds bind to pairs of cysteine residues. This binding is responsible for the toxicity of many arsenic compounds[3] Binding is reversed by 1,2-ethanedithiol (EDT), which binds tightly to arsenic compounds, as shown by the stability of FlAsH-EDT2.[4] Such strong sulfur-arsenic bond can be, again, regulated by designing a peptide domain that exhibits higher affinity toward the arsenic, such as tetracysteine motif. By modulating the distance between the two pairs of cysteine residues and the space between the arsenic centers of FlAsH-EDT2, a cooperative and entropically favored dithiol arsenic bond could be achieved.[5]
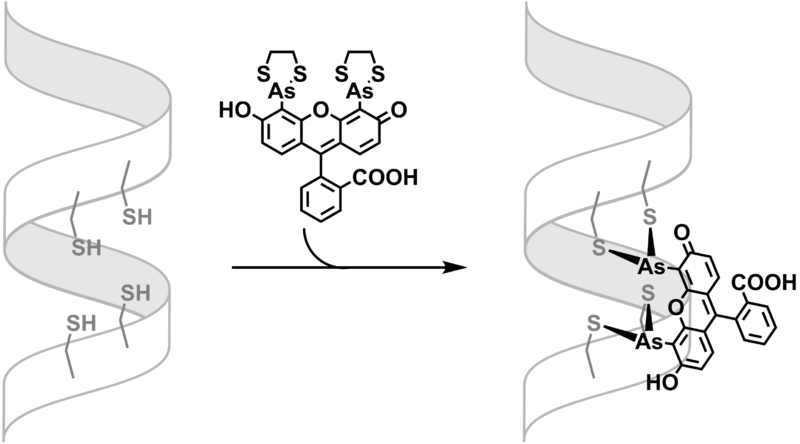
The binding of FlAsH-EDT2 is thus subject to equilibration. The FlAsH-peptide adduct formation can be favored in low concentration of EDT (<10μM) and be reversed in high concentration of EDT (>1mM).[5]
Properties
FlAsH becomes fluorescent upon the binding of tetracysteine motif. It is excited at 508 nm and emits 528 nm, a green-yellow, of free fluorescein. The quantum yield is 0.49 for 250 nM FlAsH is bound to a model tetracysteine-containing peptide in a phosphate-buffered saline at pH 7.4.[5]
Generally, FlAsH-EDT2 has 0.1-0.6 fluorescence quantum efficiencies with several μM detection limits for diffuse cytosolic tag and 30 - 80 extinction coefficients (mM)-1 cm-1. The FlAsH-peptide complex also has demonstrated fluorescence resonance energy transfer (FRET) from fluorescent proteins, such as from enhanced cyan fluorescent protein (ECFP) of Green Fluorescent Protein (GFP).[6]
Application
FlAsH-EDT2 enables less toxic and more specific fluorescent labeling that is membrane permeable. The modification of the fluorescein moiety also allows multicolor analysis. It has been proven to be a good alternative to green fluorescent proteins (GFP) with the advantage that FlAsH-EDT2 is much smaller (<1 kDa) as compared to GFPs (~30 kDa), therefore minimizing the perturbation of activity of the protein under the study.[7]
Use
In the past, FlAsH-EDT2 has been widely used to study a number of in vivo cellular events and subcellular structures in animal cells, Ebola virus matrix protein, and protein misfolding in vivo. With the electron microscopic imaging, FlAsH-EDT2 is also used to study the processes of protein trafficking in situ.[8] More recently, it was used in an extended study of plant cells like Arabidopsis and tobacco.[9]
References
- ↑ Adams, Stephen R; Tsien, Roger Y (2008). "Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins". Nature Protocols. 3 (9): 1527–34. doi:10.1038/nprot.2008.144. PMC 2843588
 . PMID 18772880.
. PMID 18772880. - ↑ Adams, R.; Tsien, R. (2008). "Preparation of the membrane-permeant biarsenicals FlAsH-EDT2 and ReAsH-EDT2 for fluorescent labeling of tetracysteine-tagged proteins". Nature Protocols. 3 (9): 1527–1534. doi:10.1038/nprot.2008.144. PMC 2843588
 . PMID 18772880.
. PMID 18772880. - ↑ Kalef, E.; Gitler, C. (1994). "Purification of Vicinal Dithiol-containing Proteins by Arsenical-Based Affinity Chromatography". Methods in Enzymology. 233: 395–403. doi:10.1016/S0076-6879(94)33046-8.
- ↑ Whittaker, V. P. (1947). "An Experimental Investigation of the 'Ring Hypothesis'of Arsenical Toxicity". Biochemical Journal. 41: 56–62. doi:10.1042/bj0410056.
- 1 2 3 Griffin, B. Albert; et al. (1998). "Live Cells Specific Covalent Labeling of Recombinant Protein Molecules Inside". Science. 281 (5374): 269–72. doi:10.1126/science.281.5374.269. PMID 9657724.
- ↑ Adams, Stephen R.; Campbell, Robert E.; Gross, Larry A.; Martin, Brent R.; Walkup, Grant K.; Yao, Yong; Llopis, Juan; Tsien, Roger Y. "New Biarsenical Ligands and Tetracysteine Motifs for Protein Labeling in Vitro and in Vivo: Synthesis and Biological Applications". Journal of the American Chemical Society. 2002 (124): 6063–6076. doi:10.1021/ja017687n.
- ↑ Thorner, Jeremy; Emr, Scott D.; Abelson, John N (2000). Methods in Enzymology. 327: 565–578. Missing or empty
|title=(help) - ↑ Gaietta, Guido; et al. (2002). "Multicolor and Electron Microscopic Imaging of Connexin Trafficking". Science. 296: 503–7. doi:10.1126/science.1068793. PMID 11964472.
- ↑ Estévez, José M.; Somerville, Chris (2006). "FlAsH-based live-cell fluorescent imaging of synthetic peptides expressed in Arabidopsis and tobacco". BioTechniques. 41: 569–574. doi:10.2144/000112264.
