Gatifloxacin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Zymar |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a605012 |
| Routes of administration |
Oral (discontinued), Intravenous (discontinued) ophthalmic |
| ATC code | J01MA16 (WHO) S01AE06 (WHO) |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Protein binding | 20% |
| Biological half-life | 7 to 14 hours |
| Identifiers | |
| |
| CAS Number |
112811-59-3 |
| PubChem (CID) | 5379 |
| DrugBank |
DB01044 |
| ChemSpider |
5186 |
| UNII |
81485Y3A9A |
| KEGG |
D08011 |
| ChEBI |
CHEBI:5280 |
| ChEMBL |
CHEMBL31 |
| NIAID ChemDB | 044913 |
| ECHA InfoCard | 100.190.526 |
| Chemical and physical data | |
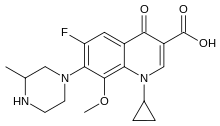
| Formula | C19H22FN3O4 |
| Molar mass | 375.394 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Gatifloxacin sold under the brand names Gatiflo, Tequin and Zymar, is an antibiotic of the fourth-generation fluoroquinolone family,[1] that like other members of that family, inhibits the bacterial enzymes DNA gyrase and topoisomerase IV. Bristol-Myers Squibb introduced Gatifloxacin in 1999 under the proprietary name Tequin for the treatment of respiratory tract infections, having licensed the medication from Kyorin Pharmaceutical Company of Japan. Allergan produces it in eye-drop formulation under the names Zymar and Zymaxid. In many countries, gatifloxacin is also available as tablets and in various aqueous solutions for intravenous therapy.
Side-effects and removal from the market
A Canadian study published in the New England Journal of Medicine in March 2006 claims Tequin can have significant side effects including dysglycemia.[2] An editorial by Dr. Jerry Gurwitz in the same issue called for the Food and Drug Administration (FDA) to consider giving Tequin a black box warning.[3] This editorial followed distribution of a letter dated February 15 by Bristol-Myers Squibb to health care providers indicating action taken with the FDA to strengthen warnings for the medication.[4] Subsequently it was reported on May 1, 2006 that Bristol-Myers Squibb would stop manufacture of Tequin, end sales of the drug after existing stockpiles were exhausted, and return all rights to Kyorin.[5]
Union Health and Family Welfare Ministry of India on 18 March 2011 banned the manufacture, sale and distribution of Gatifloxacin as it caused certain adverse side effects[6]
Contraindications
Availability
Gatifloxacin is currently available in the US and Canada only as an ophthalmic solution.
In China it is sold in tablet as well as in eye drop formulations.
Ophthalmic anti-infectives are generally well tolerated. The concentration of the drug observed following oral administration of 400 mg gatifloxacin systemically is approximately 800 times higher than that of the 0.5% Gatifloxacin eye drop. Given as an eye drop, Gatifloxacin Ophthalmic Solution 0.3% & 0.5% cause very low systemic exposures. Therefore, the systemic exposures resulting from the gatifloxacin ophthalmic solution are not likely to pose any risk for systemic toxicities.
References
- ↑ Burka JM, Bower KS, Vanroekel RC, Stutzman RD, Kuzmowych CP, Howard RS (July 2005). "The effect of fourth-generation fluoroquinolones gatifloxacin and moxifloxacin on epithelial healing following photorefractive keratectomy". Am. J. Ophthalmol. 140 (1): 83–7. doi:10.1016/j.ajo.2005.02.037. PMID 15953577.
- ↑ Park-Wyllie, Laura Y.; David N. Juurlink; Alexander Kopp; Baiju R. Shah; Therese A. Stukel; Carmine Stumpo; Linda Dresser; Donald E. Low; Muhammad M. Mamdani (March 2006). "Outpatient Gatifloxacin Therapy and Dysglycemia in Older Adults". The New England Journal of Medicine. 354 (13): 1352–1361. doi:10.1056/NEJMoa055191. PMID 16510739. Retrieved 2006-05-01. Note: publication date 30 March; available on-line 1 March
- ↑ Gurwitz, Jerry H. (March 2006). "Serious Adverse Drug Effects — Seeing the Trees through the Forest". The New England Journal of Medicine. 354 (13): 1413–1415. doi:10.1056/NEJMe068051. PMID 16510740. Retrieved 2006-05-01.
- ↑ Lewis-Hall, Freda (February 15, 2006). "Dear Healthcare Provider:" (PDF). Bristol-Myers Squibb. Retrieved May 1, 2006.
- ↑ Schmid, Randolph E. (May 1, 2006). "Drug Company Taking Tequin Off Market". Associated Press. Archived from the original on November 25, 2007. Retrieved 2006-05-01.
- ↑ "Two drugs banned". The Hindu. Chennai, India. 19 March 2011.
- ↑ Peggy Peck (2 May 2006). "Bristol-Myers Squibb Hangs No Sale Sign on Tequin". Med Page Today. Retrieved 24 February 2009.