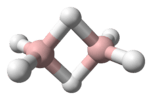Helium hydride ion
 | |
 | |
| Names | |
|---|---|
| Systematic IUPAC name
Hydridohelium(1+)[1] | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:33688 |
| ChemSpider | 21106447 |
| 2 | |
| |
| |
| Properties | |
| HeH+ | |
| Molar mass | 5.01 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
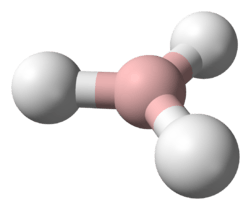
The hydrohelium(1+) cation, HeH+, also known as the helium hydride ion or helium-hydride molecular ion, is a positively charged ion formed by the reaction of a proton with a helium atom in the gas phase, first produced in the laboratory in 1925. It is isoelectronic with molecular hydrogen.[2] It is the strongest known acid, with a proton affinity of 177.8 kJ/mol.[3] It has been suggested that HeH+ should occur naturally in the interstellar medium, but it has not yet been detected.[4] It is the simplest heteronuclear ion, and is comparable with the hydrogen molecular ion, H+
2. Unlike H+
2, however, it has a permanent dipole moment, which makes its spectroscopic characterization easier.[5]
Properties
HHe+ cannot be prepared in a condensed phase, as it would protonate any anion, molecule or atom with which it were associated. However it is possible to estimate a hypothetical aqueous acidity using Hess's law:
| HHe+(g) | → | H+(g) | + He(g) | +178 kJ/mol | [3] |
| HHe+(aq) | → | HHe+(g) | +973 kJ/mol | [6] | |
| H+(g) | → | H+(aq) | −1530 kJ/mol | ||
| He(g) | → | He(aq) | +19 kJ/mol | [7] | |
| HHe+(aq) | → | H+(aq) | + He(aq) | −360 kJ/mol | |
A free energy change of dissociation of −360 kJ/mol is equivalent to a pKa of −63.
The length of the covalent bond in HeH+ is 0.772 Å.[8]
Other helium hydride ions are known or have been studied theoretically. HeH+
2, which has been observed using microwave spectroscopy,[9] has a calculated binding energy of 25.1 kJ/mol, while HeH+
3 has a calculated binding energy of 0.42 kJ/mol.[10]
The dihelium hydride cation is formed by the reaction of dihelium cation with molecular hydrogen:
- He2+ + H2 → He2H+ + H•.
He2H+ is a linear ion with hydrogen in the centre.[11]
Reactions
The helium hydride cation reacts with most substances. It has been shown to add a proton to O2, NH3, SO2, H2O, and CO2, giving O2H+, NH+
4, HSO+
2, H3O+, and HCO+
2.[11] Other molecules such as nitric oxide, nitrogen dioxide, nitrous oxide, hydrogen sulfide, methane, acetylene, ethylene, ethane, methanol and acetonitrile react but break up due to the large amount of energy produced.[11] One technique used to study reactions between organic substances and HeH+ is to make a tritium derivative of the organic compound. Decay of tritium to 3He+ followed by its extraction of a hydrogen atom yields 3HeH+ which is then surrounded by the organic material and will in turn react.[11]
Extra helium atoms can attach to HeH+ to form larger clusters such as He2H+, He3H+, He4H+, He5H+ and He6H+ which is particularly stable.[12]
Natural occurrence
The helium hydride ion is formed during the decay of tritium in the molecule HT or tritium molecule T2. Although excited by the recoil from the beta decay the molecule remains bound together.[13]
HeH+ is thought to exist in the interstellar medium, although it has not yet been unambiguously detected.[14] It is believed to be the first compound to have formed in the universe,[14] and is of fundamental importance in understanding the chemistry of the early universe.[15] This is because hydrogen and helium were almost the only types of atoms formed in Big Bang nucleosynthesis. Stars formed from the primordial material should contain HeH+, which could influence their formation and subsequent evolution. In particular, its strong dipole moment makes it relevant to the opacity of zero-metallicity stars.[14] HeH+ is also thought to be an important constituent of the atmospheres of helium-rich white dwarfs, where it increases the opacity of the gas and causes the star to cool more slowly.[16]
Several locations have been suggested as possible places HeH+ might be detected. These include cool helium stars,[14] H II regions,[17] and dense planetary nebulae[17] (in particular NGC 7027).[15] Detecting HeH+ spectroscopically is complicated by the fact that one of its most prominent spectral lines, at 149.14 μm, coincides with a doublet of spectral lines belonging to the methylidyne radical ⫶CH.[14]
HeH+ could be formed in the cooling gas behind dissociative shocks in dense interstellar clouds, such as the shocks caused by stellar winds, supernovae and outflowing material from young stars. If the speed of the shock is greater than about 90 km/s, quantities large enough to detect might be formed. If detected, the emissions from HeH+ would then be useful tracers of the shock.[18]
Neutral molecule
Unlike the helium hydride ion, the neutral helium hydride molecule is not stable in the ground state. However, it does exist in an excited state as an excimer, and its spectrum was first observed in the mid 1980s.[19][20][21]
References and notes
Unless otherwise stated, numerical data are taken from Weast, R. C. (Ed.) (1981). CRC Handbook of Chemistry and Physics (62nd Edn.). Boca Raton, FL: CRC Press. ISBN 0-8493-0462-8.
- ↑ "hydridohelium(1+) (CHEBI:33688)". Chemical Entities of Biological Interest (ChEBI). UK: European Bioinformatics Institute.
- ↑ T. R. Hogness; E. G. Lunn (1925). "The Ionization of Hydrogen by Electron Impact as Interpreted by Positive Ray Analysis". Physical Review. 26 (1): 44–55. Bibcode:1925PhRv...26...44H. doi:10.1103/PhysRev.26.44.
- 1 2 Lias, S. G.; Liebman, J. F.; Levin, R. D.; Liebman; Levin (1984). "Evaluated Gas Phase Basicities and Proton Affinities of Molecules; Heats of Formation of Protonated Molecules". Journal of Physical and Chemical Reference Data. 13 (3): 695. Bibcode:1984JPCRD..13..695L. doi:10.1063/1.555719.
- ↑ J. Fernandez; F. Martin; Martín (2007). "Photoionization of the HeH+ molecular ion". J. Phys. B: At. Mol. Opt. Phys. 40 (12): 2471–2480. Bibcode:2007JPhB...40.2471F. doi:10.1088/0953-4075/40/12/020.
- ↑ Coxon, J; Hajigeorgiou, PG (1999). "Experimental Born–Oppenheimer Potential for the X1Σ+ Ground State of HeH+: Comparison with the Ab Initio Potential". Journal of Molecular Spectroscopy. 193 (2): 306–318. Bibcode:1999JMoSp.193..306C. doi:10.1006/jmsp.1998.7740. PMID 9920707.
- ↑ Estimated to be the same as for Li+(aq) → Li+(g).
- ↑ Estimated from solubility data.
- ↑ Coyne, John P.; Ball, David W. (2009). "Alpha particle chemistry. On the formation of stable complexes between He2+ and other simple species: implications for atmospheric and interstellar chemistry". Journal of Molecular Modeling. 15 (1): 35–40. doi:10.1007/s00894-008-0371-3. PMID 18936986.
- ↑ Alan Carrington; David I. Gammie; Andrew M. Shaw; Susie M. Taylor; Jeremy M. Hutson (1996). "Observation of a microwave spectrum of the long-range He···H2+ complex". Chemical Physics Letters. 260 (3–4): 395–405. Bibcode:1996CPL...260..395C. doi:10.1016/0009-2614(96)00860-3.
- ↑ F. Pauzat and Y. Ellinger Where do noble gases hide in space?, Astrochemistry: Recent Successes and Current Challenges, Poster Book IAU Symposium No. 231, 2005 A. J. Markwick-Kemper (ed.)
- 1 2 3 4 Grandinetti, Felice (October 2004). "Helium chemistry: a survey of the role of the ionic species". International Journal of Mass Spectrometry. 237 (2-3): 243–267. doi:10.1016/j.ijms.2004.07.012.
- ↑ Grandinetti, Felice (October 2004). "Helium chemistry: a survey of the role of the ionic species". International Journal of Mass Spectrometry. 237 (2–3): 243–267. doi:10.1016/j.ijms.2004.07.012.
- ↑ F Mannone: Safety in Tritium Handling Technology Springer 1993, p 92
- 1 2 3 4 5 Engel, Elodie A.; Doss, Natasha; Harris, Gregory J.; Tennyson, Jonathan (2005). "Calculated spectra for HeH+ and its effect on the opacity of cool metal-poor stars". Monthly Notices of the Royal Astronomical Society. 357 (2): 471–477. arXiv:astro-ph/0411267
 . Bibcode:2005MNRAS.357..471E. doi:10.1111/j.1365-2966.2005.08611.x.
. Bibcode:2005MNRAS.357..471E. doi:10.1111/j.1365-2966.2005.08611.x. - 1 2 Liu, X.-W.; Barlow, M.J.; Dalgarno, A.; Tennyson, J.; Lim, T.; Swinyard, B.M.; Cernicharo, J.; Cox, P.; Baluteau, J.-P.; Pequignot, D.; Nguyen-Q-Rieu; Emery, R.J.; Clegg, P.E. (1997). "An ISO Long Wavelength Spectrometer detection of CH in NGC 7027 and an HeH+ upper limit". Monthly Notices of the Royal Astronomical Society. 290 (4): L71–L75. Bibcode:1997MNRAS.290L..71L. doi:10.1093/mnras/290.4.l71.
- ↑ Harris, G.J.; Lynas-Gray, A.E.; Miller, S.; Tennyson, J. (2004). "The Role of HeH+ in Cool Helium-rich White Dwarfs". The Astrophysical Journal. 617 (2): L143–L146. arXiv:astro-ph/0411331
 . Bibcode:2004ApJ...617L.143H. doi:10.1086/427391.
. Bibcode:2004ApJ...617L.143H. doi:10.1086/427391. - 1 2 Roberge, W.; Delgarno, A. (1982). "The formation and destruction of HeH+ in astrophysical plasmas". The Astrophysical Journal. 255: 489–496. Bibcode:1982ApJ...255..489R. doi:10.1086/159849.
- ↑ Neufeld, David A.; Dalgarno, A. (1989). "Fast molecular shocks. I – Reformation of molecules behind a dissociative shock". Astrophysical Journal. 340: 869–893. Bibcode:1989ApJ...340..869N. doi:10.1086/167441.
- ↑ Thomas Möller; Michael Beland; Georg Zimmerer (1985). "Observation of Fluorescence of the HeH Molecule". Phys. Rev. Lett. 55 (20): 2145–2148. Bibcode:1985PhRvL..55.2145M. doi:10.1103/PhysRevLett.55.2145. PMID 10032060.
- ↑ Wolfgang Ketterle, The Nobel Prize in Physics 2001
- ↑ W. Ketterle; H. Figger; H. Walther (1985). "Emission spectra of bound helium hydride". Phys. Rev. Lett. 55 (27): 2941–2944. Bibcode:1985PhRvL..55.2941K. doi:10.1103/PhysRevLett.55.2941. PMID 10032281.
J. Chem. Phys. 41, 1646 (1964)