History of fluorine
Fluorine is a relatively new element in human applications. In ancient times, only minor uses of fluorine-containing minerals existed. The industrial use of fluorite, fluorine's source mineral, was first described by early scientist Georgius Agricola in the 16th century, in the context of smelting. The name "fluorite" (and later "fluorine") derives from Agricola's invented Latin terminology. In the late 18th century, hydrofluoric acid was discovered. By the early 19th century, it was recognized that fluorine was a bound element within compounds, similar to chlorine. Fluorite was determined to be calcium fluoride.
Because of fluorine's tight bonding as well as the toxicity of hydrogen fluoride, the element resisted many attempts to isolate it. In 1886, French chemist Henri Moissan, later a Nobel Prize winner, succeeded in making elemental fluorine by electrolyzing a mixture of potassium fluoride and hydrogen fluoride. Large-scale production and use of fluorine began during World War 2 as part of the Manhattan Project. Earlier in the century, the main fluorochemicals were commercialized by the DuPont company: refrigerant gases (Freon) and polytetrafluoroethylene plastic (Teflon).
Ancient use
Some instances of ancient use of fluorite, main source mineral of fluorine, for ornamental use carvings exist. However, archeological finds are rare, perhaps in part because of the stone's softness. Two Roman cups made of Persian fluorite have been discovered and are currently exhibited at the British museum. Pliny the Elder described a soft stone from Persia used in cups that may have been fluorite.
Fluorite carvings from about 1000 AD have been discovered in the Americas in Indian burial grounds.
Early metallurgy

The word "fluorine" derives from the Latin stem of the main source mineral, fluorite, which was first mentioned in 1529 by Georgius Agricola, the "father of mineralogy". He described fluorite as a flux—an additive that helps melt ores and slags during smelting.[1][2] Fluorite stones were called schone flusse in the German of the time. Agricola, writing in Latin but describing 16th century industry, invented several hundred new Latin terms. For the schone flusse stones, he used the Latin noun fluores, "fluxes", because they made metal ores flow when in a fire. After Agricola, the name for the mineral evolved to fluorspar (still commonly used) and then to fluorite.[3][4][5]
Fluorite mineral was also described in the writings of alchemist Basilius Valentinus, supposedly in the late 15th century. However, it is alleged that "Valentinus" was a hoax as his writings were not known until about 1600.
Hydrofluoric acid

Some sources claim that the first production of hydrofluoric acid was by Heinrich Schwanhard, a German glass cutter, in 1670.[6] A peer-reviewed study of Schwanhard's writings, though, showed no specific mention of fluorite and only discussion of an extremely strong acid. It was hypothesized that this was probably nitric acid or aqua regia, both capable of etching soft glass.[7]
Andreas Sigismund Marggraf made the first definite preparation of hydrofluoric acid in 1764 when he heated fluorite with sulfuric acid in glass, which was greatly corroded by the product.[8][9] In 1771, Swedish chemist Carl Wilhelm Scheele repeated this reaction.[9][10] Scheele recognized the product of the reaction as an acid, which he called "fluss-spats-syran" (fluor-spar-acid); in English, it was known as "fluoric acid".
Recognition of the element
In 1810, French physicist André-Marie Ampère suggested that hydrofluoric acid was a compound of hydrogen with an unknown element, analogous to chlorine.[11] Fluorite was then shown to be mostly composed of calcium fluoride.[12]
Sir Humphry Davy originally suggested the name fluorine, taking the root from the name of "fluoric acid" and the -ine suffix, similarly to other halogens. This name, with modifications, came to most European languages. (Greek, Russian, and several other languages use the name ftor or derivatives, which was suggested by Ampère and comes from the Greek φθόριος (phthorios), meaning "destructive".)[13] The New Latin name (fluorum) gave the element its current symbol, F, although the symbol Fl has been used in early papers.[14] The symbol Fl is now used for the super-heavy element flerovium.[15]

Early isolation attempts
.jpg)
Progress in isolating the element was slowed by the exceptional dangers of generating fluorine: several 19th century experimenters, the "fluorine martyrs", were killed or blinded. Davy, as well as the notable French chemists Joseph Louis Gay-Lussac and Louis Jacques Thénard, experienced severe pains from inhaling hydrogen fluoride gas; Davy's eyes were damaged. Irish chemists Thomas and George Knox developed fluorite apparatus for working with hydrogen fluoride, but nonetheless were severely poisoned. Thomas nearly died and George was an invalid for three years. Belgian chemist Paulin Louyet and French chemist Jerome Nickles tried to follow the Knox work, but they died from HF poisoning even though they were aware of the dangers.[12][16]
Humphry Davy of England: poisoned, recovered. George and Thomas Knox of Ireland: both poisoned, one bedridden 3 years, recovered. P. Louyet of Belgium: poisoned, died. Jerome Nickels of Nancy, France: poisoned, died. George Gore of England: fluorine / hydrogen explosion, narrowly escaped injury. Henri Moissan of France: poisoned several times, success, but shortened life.
Initial attempts to isolate the element were also hindered by material difficulties: the extreme corrosiveness and reactivity of hydrogen fluoride (and of fluorine gas) as well as problems getting a suitable conducting liquid for electrolysis. Davy tried to electrolyze HF but had to stop because the electrodes were damaged. He then shifted to (unsuccessful) chemical reactions.
Edmond Frémy thought that passing electric current through pure hydrofluoric acid (dry HF) might work. Previously, hydrogen fluoride was only available in a water solution. Frémy therefore devised a method for producing dry hydrogen fluoride by acidifying potassium bifluoride (KHF2). Unfortunately, pure hydrogen fluoride did not pass an electric current. Frémy also tried electrolyzing molten calcium fluoride and probably produced some fluorine (since he made calcium metal at the other electrode), but he was unable to collect the gas.
English chemist George Gore also tried electrolyzing dry HF and may have made small quantities of fluorine gas in 1860. He reported an explosion after running his cell (hydrogen and fluorine recombine dramatically), but he recognized that an oxygen leak could have also caused the reaction.[6][12][16]
Moissan

French chemist Henri Moissan, formerly one of Frémy's students, continued the search. After trying many different approaches, he built on Frémy and Gore's earlier attempts by combining potassium bifluoride and hydrogen fluoride. The resultant solution conducted electricity. Moissan also constructed especially corrosion-resistant equipment: containers crafted from a mixture of platinum and iridium (more chemically resistant than pure platinum) with fluorite stoppers.[17][16]
After 74 years of effort by many chemists, on 26 June 1886, Moissan isolated elemental fluorine.[6][18] Moissan's report to the French Academy of making fluorine showed appreciation for the feat: "One can indeed make various hypotheses on the nature of the liberated gas; the simplest would be that we are in the presence of fluorine."[17]
Moissan's 1887 publication documents reaction attempts of fluorine gas with several substances: sulfur (flames), hydrogen (explosion), carbon (no reaction), etc. Later, Moissan devised a less expensive apparatus for making fluorine: copper equipment coated with copper fluoride.
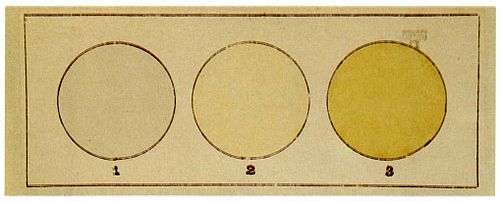
Moissan also constructed special apparatus—5m long platinum tubes with fluorite windows—to determine the slight yellow color of fluorine gas. (The gas appears transparent in small tubes or when allowed to escape. The color observation was not repeated until the 1980s, when his result was confirmed.)
In 1906, two months before his death, Moissan received the Nobel Prize in chemistry.[19][20][21] The citation:[16][note 1]
...in recognition of the great services rendered by him in his investigation and isolation of the element fluorine...The whole world has admired the great experimental skill with which you have studied that savage beast among the elements.
Development
During the 1930s and 1940s, the DuPont company commercialized organofluorine compounds at large scales. Following trials of chlorofluorcarbons as refrigerants by researchers at General Motors, DuPont developed large-scale production of Freon-12. DuPont and GM formed a joint venture in 1930 to market the new product; in 1949 DuPont took over the business. Freon proved to be a marketplace hit, rapidly replacing earlier, more toxic, refrigerants and growing the overall market for kitchen refrigerators.[9][22][23][24]
In 1938, polytetrafluoroethylene (Teflon) was discovered by accident by a recently hired DuPont PhD, Roy J. Plunkett. While working with a cylinder of tetrafluoroethylene, he was unable to release the gas, although the weight had not changed. Scraping down the container, he found white flakes of a polymer new to the world. Tests showed the substance was resistant to corrosion from most substances and had better high temperature stability than any other plastic. By early 1941, a crash program was making commercial quantities.[9][23][25]

Large-scale productions of elemental fluorine began during World War II. Germany used high-temperature electrolysis to produce tons of chlorine trifluoride, a compound planned to be used as an incendiary.[26] The Manhattan project in the United States produced even more fluorine for use in uranium separation. Gaseous uranium hexafluoride was used to separate uranium-235, an important nuclear explosive, from the heavier uranium-238 in diffusion plants. Because uranium hexafluoride releases small quantities of corrosive fluorine, the separation plants were built with special materials. All pipes were coated with nickel; joints and flexible parts were fabricated from Teflon.[23]
In 1958, a DuPont research manager in the Teflon business, Bill Gore, left the company because of its unwillingness to develop Teflon as wire-coating insulation. Gore's son Robert found a method for solving the wire-coating problem and the company W. L. Gore and Associates was born.[27] In 1969, Robert Gore developed an expanded polytetrafluoroethylene (ePTFE) membrane which led to the large Gore-Tex business in breathable rainwear. The company developed many other uses of PTFE.[28]
In the 1970s and 1980s, concerns developed over the role chlorofluorocarbons play in damaging the ozone layer. By 1996, almost all nations had banned chlorofluorocarbon refrigerants and commercial production ceased. Fluorine continued to play a role in refrigeration though: hydrochlorofluorocarbons (HCFCs) and hydrofluorocarbons (HFCs) were developed as replacement refrigerants.[29][30]

Notes
- ↑ Moissan's Nobel also honored his invention of the electric arc furnace
Citations
- ↑ Greenwood & Earnshaw 1998, p. 790.
- ↑ Senning, Alexander (2007). Elsevier's dictionary of chemoetymology: The whies and whences of chemical nomenclature and terminology. Elsevier. p. 149. ISBN 978-0-444-52239-9. Retrieved 7 May 2011.
- ↑ Norwood, Charles J.; Fohs, Julius F. (1907). "Fluorspar and its Occurrence". Kentucky geological survey Bulletin 9: Fluorspar deposits of Kentucky. Globe Printing Company. p. 52.
- ↑ Agricola, Georgius (1912). De Re Metallica. trans. Hoover, Herbert Clark; Hoover, Henry Lou. pp. preface, 380–381 (3, 416–417 in linked viewer).
- ↑ Greenwood & Earnshaw 1998, p. 109.
- 1 2 3 Asimov, Isaac (1966). The noble gases. Basic Books. p. 162. ISBN 978-0-465-05129-8.
- ↑ Partington, J. R. (1923). "The early history of hydrofluoric acid". Memoirs and Proceedings of the Manchester Literary and Philosophical Society. 67 (6): 73–87.
- ↑ Marggraf, Andreas Sigismun (1770). "Observation concernant une volatilisation remarquable d'une partie de l'espece de pierre, à laquelle on donne les noms de flosse, flüsse, flus-spaht, et aussi celui d'hesperos; laquelle volatilisation a été effectuée au moyen des acides" [Observation of a remarkable volatilization of part of a type of stone to which one gives the name flosse, flüsse, flus-spaht, as well as that of hesperos; which volatilization was effected by means of acids]. Mémoires de l'Académie royale des sciences et belles-lettres (in French): 3–11.
- 1 2 3 4 Kirsch, Peer (2004). "Fluorine". Modern fluoroorganic chemistry: Synthesis, reactivity, applications. pp. 3–10. ISBN 978-3-527-30691-6. Retrieved 7 May 2011.
- ↑ Scheele, Carl Wilhelm (1771). "Undersŏkning om fluss-spat och dess syra" [Investigation of fluorite and its acid]. Kungliga Svenska Vetenskapsademiens Handlingar (Proceedings of the Royal Swedish Academy of Science) (in Swedish). 32: 129–138.
- ↑ Ampere, André-Marie (1816). "Suite d'une classification naturelle pour les corps simples". Annales de chimie et de physique (in French). 2: 1–5. Retrieved 7 May 2011.
- 1 2 3 Weeks, Mary Elvira (1932). "The discovery of the elements. XVII. The halogen family". Journal of Chemical Education. 9 (11): 1915–1939. Bibcode:1932JChEd...9.1915W. doi:10.1021/ed009p1915.
- ↑ "09 Fluorine". elements.vanderkrogt.net. Retrieved 24 January 2012.
- ↑ Storer, Frank Humphreys (1864). First outlines of a dictionary of solubilities of chemical substances. Cambridge University Press. pp. 278–280. ISBN 978-1-176-62256-2.
- ↑ "Element 114 is named flerovium and element 116 is named livermorium". IUPAC. 2012-05-30. Retrieved 1 June 2012.
- 1 2 3 4 "Fluorine, an obsession with a tragic past"; Toon, Richard; Education in Chemistry, September 2011, 148-151; http://www.rsc.org/images/EiC_Sept2011_fluorine_tcm18-207109.pdf
- 1 2 Greenwood & Earnshaw 1998, pp. 789–791.
- ↑ Moissan, Henry (1886). "Action d'un courant électrique sur l'acide fluorhydrique anhydre". Comptes rendus hebdomadaires des séances de l'Académie des sciences (in French). 102: 1543–1544. Retrieved 7 May 2011.
- ↑ "The Nobel Prize in chemistry 1906". nobelprize.org. Retrieved 7 July 2009.
- ↑ Greenwood & Earnshaw 1998, p. 791.
- ↑ James, Laylin K. (1993). Nobel laureates in chemistry, 1901–1992. Chemical Heritage Foundation. p. 35. ISBN 978-0-8412-2690-6.
- ↑ Hounshell & Smith 1988, p. 156.
- 1 2 3 Okazoe, Takashi (2009). "Overview on the history of organofluorine chemistry from the viewpoint of material industry" (PDF). Proceedings of the Japan Academy, Series B. 85 (8): 276–289. Bibcode:2009PJAB...85..276O. doi:10.2183/pjab.85.276.
- ↑ "Freon". dupont.com. Retrieved 10 November 2012.
- ↑ Hounshell & Smith 1988, p. 157.
- ↑ Meyer, Eugene (1977). Chemistry of hazardous materials. Prentice Hall (1st ed.). p. 111. ISBN 9780131292390.
- ↑ Hounshell & Smith 1988, p. 489.
- ↑ Harrington, Ann (2003). "Who's afraid of a new product? Not W.L. Gore. It has mastered the art of storming completely different businesses". CNN Money (Fortune Magazine). Retrieved 21 January 2012.
- ↑ U.S. Environmental Protection Agency (2008). "Ozone depletion glossary". Retrieved 3 September 2008.
- ↑ U.S. Environmental Protection Agency (2006). "Brief questions and answers on ozone depletion | Ozone layer protection". Retrieved 8 November 2011.
Indexed references
External links
- (French) Research in the isolation of Fluorine, 1887 report by Moissan with drawings, on the French Wikisource.