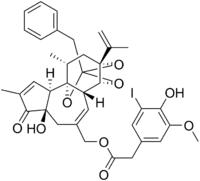
Iodoresiniferatoxin
 | |
| Names | |
|---|---|
| Other names
5-Iodoresiniferatoxin | |
| Identifiers | |
| 535974-91-5 | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL595069 |
| PubChem | 6324614 |
| |
| Properties | |
| C37H39IO9 | |
| Molar mass | 754.61 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Iodoresiniferatoxin (I-RTX) is a strong competitive antagonist of the Transient Receptor Potential Vanilloid 1 (TRPV1) receptor. I-RTX is derived from resiniferatoxin (RTX).
Etymology
I-RTX is an iodinated form of resiniferatoxin, which is produced by the Moroccan succulent Euphorbia resinifera. I-RTX is iodinated at the 5-position and is therefore also called 5-iodoresiniferatoxin.
Sources
I-RTX can be prepared from RTX by electrophilic aromatic substitution.[1] Iodide substitutes the 5-position.
Chemistry
Iodination of RTX at the 5-position changes the toxin from an TRPV1-receptor agonist into an TRPV1-receptor antagonist, with only a little lower affinity for TRPV1 than RTX.[2][3][4]
As RTX, I-RTX belongs to the daphnane family of molecules.[5]
The substance is soluble in DMSO and in ethanol.[6]
Binding of I-RTX is dependent upon temperature and pH value, as has been shown in research with HEK 293 cells expressing TRPV1. The optimal pH value is around 7.8 to 8.0 and binding increases markedly with temperature up to 37 °C and then decreases at higher temperatures.[2]
Target
Initially, iodoresiniferatoxin was thought to be a competitive antagonist of the TRPV1 receptor with high affinity (Kd = 4.3 ± 0.9 nM to HEK 293/VR1 and Kd = 4.2 ± 1.0 nM to rat spinal cord membranes),[2] but recent research indicated also partial transient agonistic characteristics in the thermoregulatory system in mice, especially in higher concentrations ranging from 1 to 30 µM.[6]
The TRPV1 receptor encodes a protein of 838 amino acids forming a calcium-permeable channel that is activated by capsaicin but also by noxious heat and low extracellular pH.[7] TRPV1 receptors are expressed in many systems in the central and peripheral nervous system, and have a particularly important role in signal conduction in afferent pain pathways.[8]
Mode of action
The proposed molecular mode of action of TRPV1 antagonists is blocking of the channel pore.[8] Several studies using either capsaicin, pH values < 6 or heat as the agonist have shown that I-RTX works as a strong competitive TRPV1 antagonist in vitro.[2][3][4]
Recent studies also revealed partial TRPV1-agonist-like effects of I-RTX in the thermoregulatory system in mice, increasing intracellular Ca2+ concentrations. It also exerts weak, partial agonism on recombinant TRPV1 in vitro.[6] This agonistic effect could be due to metabolization whereby I-RTX would be deiodinated, converting it into RTX with its respective characteristics.[8] In vivo, I-RTX showed analgesic activity in the capsaicin pain test.[2] I-RTX thus is able to block TRPV1 mediated nociceptive and neurogenic inflammatory responses.[3][4]
Toxicity
In mice, I-RTX induces dose-dependent hypothermia in vivo. A statistically significant difference was reported at doses > 0.1 µmol/kg. The maximal effect was found with a dose of 1 µmol/kg, 60 to 100 minutes after administration. No lethality has been reported in this study.[6]
Therapeutic use
Clinical research is done on the use of I-RTX as an analgesic agent, although several drawbacks have been mentioned: complex chemical structure, high costs of production, and relatively unfavourable pharmacokinetic characteristics.[4]
References
- ↑ Hunter, W.M.; Greenwood, F.C. (1962). "Preparation of iodine-131 labeled human growth hormone of high specific activity". Nature. 194 (4827): 495–496. doi:10.1038/194495a0. PMID 14450081.
- 1 2 3 4 5 Wahl, P; Foged, C; Tullin, S; Thomsen, C (2001). "Iodo-resiniferatoxin, a new potent vanilloid receptor antagonist.". Molecular Pharmacology. 59 (1): 9–15. PMID 11125018.
- 1 2 3 Seabrook, GR; Sutton, KG; Jarolimek, W; Hollingworth, GJ; Teague, S; Webb, J; Clark, N; Boyce, S; et al. (2002). "Functional properties of the high-affinity TRPV1 (VR1) vanilloid receptor antagonist (4-hydroxy-5-iodo-3-methoxyphenylacetate ester) iodo-resiniferatoxin". The Journal of Pharmacology and Experimental Therapeutics. 303 (3): 1052–60. doi:10.1124/jpet.102.040394. PMID 12438527.
- 1 2 3 4 Rigoni, M; Trevisani, M; Gazzieri, D; Nadaletto, R; Tognetto, M; Creminon, C; Davis, JB; Campi, B; et al. (2003). "Neurogenic responses mediated by vanilloid receptor-1 (TRPV1) are blocked by the high affinity antagonist, iodo-resiniferatoxin". British Journal of Pharmacology. 138 (5): 977–85. doi:10.1038/sj.bjp.0705110. PMC 1573721
 . PMID 12642400.
. PMID 12642400. - ↑ Seiple, I.B. (2007). Daphane, tigliane, ingenane and diterpenes, Retrieved 10 October 2009, from
- 1 2 3 4 Shimizu, I; Iida, T; Horiuchi, N; Caterina, MJ (2005). "5-Iodoresiniferatoxin evokes hypothermia in mice and is a partial transient receptor potential vanilloid 1 agonist in vitro". The Journal of Pharmacology and Experimental Therapeutics. 314 (3): 1378–85. doi:10.1124/jpet.105.084277. PMID 15947039.
- ↑ Caterina, MJ; Schumacher, MA; Tominaga, M; Rosen, TA; Levine, JD; Julius, D (1997). "The capsaicin receptor: a heat-activated ion channel in the pain pathway". Nature. 389 (6653): 816–24. doi:10.1038/39807. PMID 9349813.
- 1 2 3 Premkumar, LS; Sikand, P (2008). "TRPV1: A Target for Next Generation Analgesics". Current neuropharmacology. 6 (2): 151–63. doi:10.2174/157015908784533888. PMC 2647151
 . PMID 19305794.
. PMID 19305794.