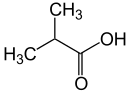
Isobutyric acid
Isobutyric acid[1]
|
|
| Names |
| IUPAC name
2-Methylpropanoic acid |
| Other names
Isobutyric acid
2-Methylpropionic acid
Isobutanoic acid |
| Identifiers |
| |
79-31-2  Y Y |
| 3D model (Jmol) |
Interactive image |
| ChEBI |
CHEBI:16135  Y Y |
| ChEMBL |
ChEMBL108778  Y Y |
| ChemSpider |
6341  Y Y |
| DrugBank |
DB02531  N N |
| ECHA InfoCard |
100.001.087 |
| |
1060 |
| KEGG |
C02632  Y Y |
| UNII |
8LL210O1U0  Y Y |
InChI=1S/C4H8O2/c1-3(2)4(5)6/h3H,1-2H3,(H,5,6)  Y YKey: KQNPFQTWMSNSAP-UHFFFAOYSA-N  Y YInChI=1/C4H8O2/c1-3(2)4(5)6/h3H,1-2H3,(H,5,6) Key: KQNPFQTWMSNSAP-UHFFFAOYAB
|
| |
| Properties |
| |
C4H8O2 |
| Molar mass |
88.11 g/mol |
| Density |
0.9697 g/cm3 at 0 °C |
| Melting point |
−47 °C (−53 °F; 226 K) |
| Boiling point |
155 °C (311 °F; 428 K) |
| Acidity (pKa) |
4.86[2] |
| Hazards |
| NFPA 704 |
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). |
 N verify (what is N verify (what is  Y Y N ?) N ?) |
| Infobox references |
|
|
Isobutyric acid, also known as 2-methylpropanoic acid, is a carboxylic acid with structural formula (CH3)2-CH-COOH. It is found in the free state in carobs (Ceratonia siliqua), in vanilla and in the root of Arnica dulcis, and as an ethyl ester in croton oil. Isobutyric acid is an isomer of n-butyric acid; they have the same chemical formula C4H8O2 but a different structure.
Production
Isobutyric acid may be artificially prepared by the hydrolysis of isobutyronitrile with alkalis, by the oxidation of isobutanol with potassium dichromate and sulfuric acid,[3] or by the action of sodium amalgam on methacrylic acid. Isobutyric acid is a liquid under common conditions with a somewhat unpleasant smell. It boils at 155 °C and has a specific gravity is 0.9697 (0 °C). When heated with a chromic acid solution to 140 °C, it decomposes to form carbon dioxide and acetone. Alkaline potassium permanganate oxidizes it to α-hydroxyisobutyric acid, (CH3)2-C(OH)-COOH. Its salts are more soluble in water than those of butyric acid.
Biological production
Isobutyric acid can also be manufactured commercially using engineered bacteria using a sugar feedstock.[4]
Isobutyric acid is a retained trivial name under the IUPAC rules.[5]
References
- ↑ Merck Index, 11th Edition, 5039
- ↑ Bjerrum, J., et al. Stability Constants, Chemical Society, London, 1958.
- ↑ I. Pierre and E. Puchot (1873). "New Studies on Valerianic Acid and its Preparation on a Large Scale". Ann. Chim. Phys. 28: 366.
- ↑ "Biological pathways to produce methacrylate".
- ↑ Panico R, Powell WH, Richer JC, eds. (1993). "Recommendation R-9.1". A Guide to IUPAC Nomenclature of Organic Compounds. IUPAC/Blackwell Science. ISBN 0-632-03488-2.
 This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "article name needed". Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "article name needed". Encyclopædia Britannica (11th ed.). Cambridge University Press.
|
|---|
|
Receptor
(ligands) | |
- PAMs: Alcohols (e.g., brometone, chlorobutanol (chloretone), ethanol, tert-butanol (2M2P), tribromoethanol, trichloroethanol, trifluoroethanol)
- Alkylbenzene sulfonate
- Anandamide
- Barbiturates (e.g., pentobarbital, sodium thiopental)
- Chlormethiazole
- D12-116
- Dihydropyridines (e.g., nicardipine)
- Etomidate
- Ginseng constituents (e.g., ginsenosides (e.g., ginsenoside-Rf))
- Glutamic acid (glutamate)
- Ivermectin
- Ketamine
- Neuroactive steroids (e.g., alfaxolone, pregnenolone (eltanolone), pregnenolone acetate, minaxolone, Org 20599)
- Nitrous oxide
- Penicillin G
- Propofol
- Tamoxifen
- Tetrahydrocannabinol
- Triclofos
- Tropeines (e.g., atropine, bemesetron, cocaine, LY-278584, tropisetron, zatosetron)
- Volatiles/gases (e.g., chloral hydrate, chloroform, desflurane, diethyl ether (ether), enflurane, halothane, isoflurane, methoxyflurane, sevoflurane, toluene, trichloroethane (methyl chloroform), trichloroethylene)
- Xenon
- Zinc
- Antagonists: 2-Aminostrychnine
- 2-Nitrostrychnine
- 4-Phenyl-4-formyl-N-methylpiperidine
- αEMBTL
- Bicuculline
- Brucine
- Cacotheline
- Caffeine
- Colchicine
- Colubrine
- Cyanotriphenylborate
- Dendrobine
- Diaboline
- Endocannabinoids (e.g., 2-AG, anandamide (AEA))
- Gaboxadol (THIP)
- Gelsemine
- iso-THAZ
- Isobutyric acid
- Isonipecotic acid
- Isostrychnine
- Laudanosine
- N-Methylbicuculline
- N-Methylstrychnine
- N,N-Dimethylmuscimol
- Nipecotic acid
- Pitrazepin
- Pseudostrychnine
- Quinolines (e.g., 4-hydroxyquinoline, 4-hydroxyquinoline-3-carboxylic acid, 5,7-CIQA, 7-CIQ, 7-TFQ, 7-TFQA)
- RU-5135
- Sinomenine
- Strychnine
- Thiocolchicoside
- Tutin
- NAMs: Amiloride
- Benzodiazepines (e.g., bromazepam, clonazepam, diazepam, flunitrazepam, flurazepam)
- Corymine
- Cyanotriphenylborate
- Daidzein
- Dihydropyridines (e.g., nicardipine, nifedipine, nitrendipine)
- Furosemide
- Genistein
- Ginkgo constituents (e.g., bilobalide, ginkgolides (e.g., ginkgolide A, ginkgolide B, ginkgolide C, ginkgolide J, ginkgolide M))
- Imipramine
- NBQX
- Neuroactive steroids (e.g., 3α-androsterone sulfate, 3β-androsterone sulfate, deoxycorticosterone, DHEA sulfate, pregnenolone sulfate, progesterone)
- Opioids (e.g., codeine, dextromethorphan, dextrorphan, levomethadone, levorphanol, morphine, oripavine, pethidine, thebaine)
- Picrotoxin (i.e., picrotin and picrotoxinin)
- PMBA
- Riluzole
- Tropeines (e.g., bemesetron, LY-278584, tropisetron, zatosetron)
- Verapamil
- Zinc
|
|---|
|
|---|
|
Transporter
(blockers) | |
|---|
|
| Others | |
|---|
|
See also: GABAergics • GHBergics • Glutamatergics |
![]() This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "article name needed". Encyclopædia Britannica (11th ed.). Cambridge University Press.
This article incorporates text from a publication now in the public domain: Chisholm, Hugh, ed. (1911). "article name needed". Encyclopædia Britannica (11th ed.). Cambridge University Press.