Ketoacyl synthase
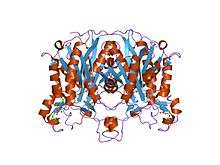
A keto-synthase (KS) is a domain of polyketide synthases with a thiol (SH) group on a cysteine side-chain. Every polyketide synthase module has an acyl carrier protein (ACP) and a ketosynthase domain that collaborate to catalyze the chain elongation.[2]
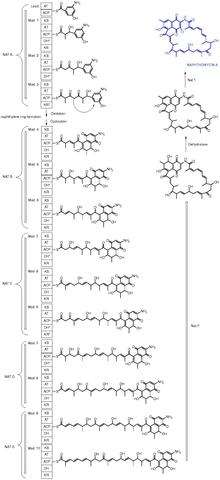
Molecules produced through an enzyme with a KS domain
3,5-Dihydroxy-4-isopropyl-trans-stilbene is a bacterial stilbenoid produced in Photorhabdus bacterial symbionts of Heterorhabditis nematodes. It is an example of a product of an alternative ketosynthase-directed stilbenoids biosynthesis pathway.[3]
Callystatin A is a polyketide natural product from the leptomycin family of antibiotics. It was first isolated in 1997 from the marine sponge Callyspongia truncata which was collected from the Goto Islands in the Nagasaki Prefecture of Japan by the Kobayashi group.
Cervimycin is a glycoside antibiotic against methicillin-resistant Staphylococcus aureus (MRSA) produced by Streptomyces tendae.[4]
Enzymes with the domain
- 6-Deoxyerythronolide B synthase (DEBS), a Type 1 polyketide synthase found in Saccharopolyspora erythraea and responsible for the synthesis of the macrolide ring which is the precursor of the antibiotic erythromycin
See also
References
- ↑ Pan, Hu; Tsai, Shiou-Chuan; Meadows, Eric S.; Miercke, Larry J.W.; Keatinge-Clay, Adrian T.; O'Connell, Joe; Khosla, Chaitan; Stroud, Robert M. (2002). "Crystal Structure of the Priming β-Ketosynthase from the R1128 Polyketide Biosynthetic Pathway". Structure. 10 (11): 1559–68. doi:10.1016/S0969-2126(02)00889-4. PMID 12429097.
- ↑ Kapur, S.; Chen, A. Y.; Cane, D. E.; Khosla, C. (2010). "Molecular recognition between ketosynthase and acyl carrier protein domains of the 6-deoxyerythronolide B synthase". Proceedings of the National Academy of Sciences. 107 (51): 22066. doi:10.1073/pnas.1014081107.
- ↑ Joyce SA, Brachmann AO, Glazer I, Lango L, Schwär G, Clarke DJ, Bode HB (2008). "Bacterial biosynthesis of a multipotent stilbene". Angew Chem Int Ed Engl. 47 (10): 1942–5. doi:10.1002/anie.200705148. PMID 18236486.
- ↑ Bretschneider, Tom; Zocher, Georg; Unger, Michelle; Scherlach, Kirstin; Stehle, Thilo; Hertweck, Christian (2011). "A ketosynthase homolog uses malonyl units to form esters in cervimycin biosynthesis". Nature Chemical Biology. 8 (2): 154–61. doi:10.1038/nchembio.746. PMID 22179067.
External links
- Ketosynthase from Streptomyces griseus at uniprot.org