Lifting gas
Because of Archimedes' principle, a lifting gas is required for aerostats to create buoyancy. Its density is lower than that of air (about 1.29 kg/m3, 1.29 g/L). Only certain lighter than air gases are suitable as lifting gases.
Gases theoretically suitable for lifting
Hot air
Heated air is frequently used in recreational ballooning. According to the Ideal gas law, an amount of gas (and also a mixture of gases such as air) expands as it is heated. As a result, a certain volume of gas has a lower weight as the temperature is higher. The average temperature of air in a hot air balloon is about 212 °F (100 °C).
Hydrogen
Hydrogen, being the lightest existing gas (14 times less dense than air), seems to be the most appropriate gas for lifting. But hydrogen has several disadvantages:
- Hydrogen is extremely flammable. Some countries have banned the use of hydrogen as a lift gas for commercial vehicles but it is allowed for recreational free ballooning in the US and Germany. The Hindenburg disaster is frequently cited as an example of the hydrogen safety risks posed by hydrogen. The high cost of helium (compared to hydrogen) has led researchers to re-investigate the safety issues of using hydrogen as a lift gas: with good engineering and good handling practices, the risks can be significantly reduced.
- Because the hydrogen molecule is very small, it can easily diffuse through many materials, so that the balloon will deflate quickly. That's one reason why many hydrogen or helium filled balloons are constructed out of Mylar. (BoPET)
Helium
Helium is the second lightest gas. For that reason, it is an attractive gas for lifting as well. A major advantage is that this gas is noncombustible. But the use of helium has some disadvantages, too:
- The same diffusion problem as above described with hydrogen;
- Helium is expensive.
- Although abundant in the universe, helium is very scarce on Earth. The only commercially viable reserves are a few natural gas wells, mostly in the US, that trapped it from the slow alpha decay of radioactive materials within Earth. By human standards helium is a non-renewable resource that cannot be practically manufactured from other materials. When released into the atmosphere, e.g., when a helium-filled balloon leaks or bursts, helium eventually escapes into the atmosphere and is lost.
Steam / water vapor
The gaseous state of water is lighter than air, incombustible and much cheaper than helium. The concept of using steam for lifting is therefore already 200 years old. The biggest challenge has always been to make a material that can resist it. In 2003, a university team in Berlin, Germany, has successfully made a 150 °C steam lifted balloon.[1] However, such a design is generally impractical due to high boiling point and condensation.
Ammonia
Ammonia is sometimes used to fill weather balloons. Due to its high boiling point (compared to helium and hydrogen), ammonia could potentially be refrigerated and liquefied aboard an airship to reduce lift and add ballast (and returned to a gas to add lift and reduce ballast). Ammonia gas is relatively heavy, poisonous, and an irritant.
Methane
Methane, the main component of natural gas, is sometimes used as a lift gas when hydrogen and helium are not available. It has the advantage of not leaking through balloon walls as rapidly as the smaller molecules of hydrogen and helium. However, methane is highly flammable and like hydrogen is not appropriate for use in passenger-carrying airships. It is also relatively dense and a potent greenhouse gas.
Coal gas
In the past, coal gas, a mixture of hydrogen, carbon monoxide and other gases, was also used in balloons. It was widely available and cheap; the down side was a higher density (reducing lift) and the high toxicity of the carbon monoxide.
Neon
Neon is lighter than air and could lift a balloon. Like helium, it is incombustible. However, it is rare on Earth and expensive, and is among the heavier lifting gases.
Nitrogen
Pure nitrogen has the advantage that it is inert and abundantly available, because it is the major component of air. However, because nitrogen is only 3% lighter than air, it is not an obvious choice for a lifting gas. Nevertheless, an aerogel named SEAgel (Safe Emulsion agar gel) has been produced that floats in air if it is filled with pure nitrogen.
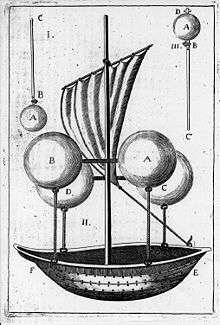
Vacuum
Theoretically, an aerostatic vehicle could be made to use a vacuum or partial vacuum. As early as 1670, over a century before the first manned hot-air balloon flight,[2] the Italian monk Francesco Lana de Terzi envisioned a ship with four vacuum spheres.
In a theoretically perfect situation with weightless spheres, a 'vacuum balloon' would be 7% lighter than a hydrogen-filled balloon, and 16% lighter than a helium-filled one. However, because the walls of the balloon must be able to remain rigid without imploding, the balloon is impractical to construct with all known materials. Despite that, sometimes there is discussion on the topic.[3]
Plasma
Another medium that in theory could be used is a plasma: Ions repelling each other could give a pressure intermediate between vacuum and hydrogen and hence that counteracts the atmospheric pressure. The energy and the containment requirements are extremely impractical, so that it may only be interesting for science fiction.
Combinations
It is also possible to combine some of the above solutions. A well-known example is the Rozière balloon which combines a core of helium with an outer shell of hot air.
Hydrogen versus helium
Hydrogen and helium are the most commonly used lift gases. Although helium is twice as heavy as (diatomic) hydrogen, they are both significantly lighter than air, making this difference negligible.
The lifting power in air of hydrogen and helium can be calculated using the theory of buoyancy as follows:
The density at sea-level and 0 °C for air and each of the gases is:
Thus helium is almost twice as dense as hydrogen. However, buoyancy depends upon the difference of the densities (ρgas) − (ρair) rather than upon their ratios. Thus the difference in buoyancies is about 8%, as seen from the buoyancy equation:
- FB=(ρair - ρgas) * g * V
- Where FB = Buoyant force (in Newton); g = gravitational acceleration = 9.8066 m/s² = 9.8066 N/kg; V = volume (in m³).
Therefore, the amount of mass that can be lifted by hydrogen in air at sea level, equal to the density difference between hydrogen and air, is:
- (1.292 - 0.090) kg/m3 = 1.202 kg/m3
and the buoyant force for one m³ of hydrogen in air at sea level is:
- 1 m3 * 1.202 kg * 9.8 N/kg= 11.8 N
Therefore, the amount of mass that can be lifted by helium in air at sea level is:
- (1.292 - 0.178) kg/m3 = 1.114 kg/m3
and the buoyant force for one m³ of helium in air at sea level is:
- 1 m3 * 1.114 kg/m³ * 9.8 N/kg= 10.9 N
Thus hydrogen's additional buoyancy compared to helium is:
- 11.8 / 10.9 ≈ 1.08, or approximately 8.0%
This calculation is at sea level at 0 °C. For higher altitudes, or higher temperatures, the amount of lift will decrease proportionally to the air density, but the ratio of the lifting capability of hydrogen to that of helium will remain the same. This calculation does not include the mass of the envelope need to hold the lifting gas.

High-altitude ballooning
At higher altitude, the air pressure is lower and therefore the pressure inside the balloon is lower. That means that while the mass of lifting gas and mass of displaced air for a given lift are the same as at lower altitude, the volume of the balloon is much greater.
A balloon that is designed to lift to extreme heights (stratosphere), must be able to expand enormously in order to displace the required amount of air. That is why such balloons seem almost empty at launch, as can be seen in the photo.
A different approach for high altitude ballooning, especially used for long duration flights is the superpressure balloon.
Submerged balloons
Because of the enormous density difference between water and gases (water is about 1,000 times more dense than most gases), the lifting power of underwater gases is very strong. The type of gas used is largely inconsequential because the relative differences between gases is negligible in relation to the density of water. However, some gases can liquefy under high pressure, leading to an abrupt loss of buoyancy.
A submerged balloon that rises will expand or even explode because of the strong pressure reduction, unless gas is able to escape continuously during the ascent or the balloon is strong enough to withstand the change in pressure.
Balloons on other celestial bodies
A balloon can only have buoyancy if there is a medium that has a higher average density than the balloon itself.
- Balloons cannot work on the Moon because it has almost no atmosphere.
- Mars has a very thin atmosphere – the pressure is only 1/160th of earth atmospheric pressure – so a huge balloon would be needed even for a tiny lifting effect. Overcoming the weight of such a balloon would be difficult, but several proposals to explore Mars with balloons have been made.[4]
- Venus has a CO2 atmosphere at the surface. Because CO2 is about 50% more dense than Earth air, ordinary Earth air could be a lifting gas on Venus. This has led to proposals for a human habitat that would float in the atmosphere of Venus at an altitude where both the pressure and the temperature are earthlike. In 1985, the Soviet Vega program sent two balloons to float in Venus' atmosphere at 54 km altitude.
- Titan, Saturn's largest moon, has a dense atmosphere of mostly nitrogen that is appropriate for ballooning. A use of Aerobots was proposed on Titan. Also the Titan Saturn System Mission included a balloon to circumnavigate Titan.
See also
References
- ↑ "HeiDAS UH – Ein Heissdampfaerostat mit ultra-heiss-performance" (PDF). Aeroix.de. Retrieved 2012-10-21.
- ↑ Tom D. Crouch (2009). Lighter Than Air
- ↑ Sean A. Barton (21 October 2009). "Stability Analysis of an Inflatable Vacuum Chamber". Arxiv.org. doi:10.1115/1.2912742. Retrieved 2012-10-21.
- ↑ "Exploring Mars With Balloons". Spacedaily.com. Retrieved 2012-10-21.