Lymphopoiesis
Lymphopoiesis (lĭm'fō-poi-ē'sĭs) (or lymphocytopoiesis) is the generation of lymphocytes, one of the five types of white blood cell (WBC).[1] It is more formally known as lymphoid hematopoiesis.
Pathosis in lymphopoiesis leads to any of various lymphoproliferative disorders, such as the lymphomas and lymphoid leukemias.
| Lymphopoiesis Glossary |
|---|
| • antigen any molecule that can provoke an immune defense |
| • B cells lymphocytes that ultimately produce antibodies |
| • bone marrow the center of bones capable of producing all red and white blood cells in the adult |
| • cortex the outer portion of any organ |
| • cytoplasm the portion of a cell between the nucleus and the membrane |
| • differentiation permanent changes to a cell developing over time and with cell division |
| • granules grains found in many white blood cells, composed of defensive chemicals |
| • hematopoietic that which gives rise to any blood cell type |
| • lineage a type of cell and its descendants by division and differentiation |
| • lymphocytes a special 'lineage' of WBC |
| • macrophages myeloid descendants (some may be lymphoid) with 'eating' abilities, also cooperate with lymphocytes |
| • myeloid ancestors of WBCs with granules and also of macrophages |
| • T Cells "management" lymphocytes for immunity |
| • (WBC) White Blood Cell in contrast to the much more common Red Blood Cell; responsible for defense |
Terminology
Lymphocytes are considered to be of the lymphoid lineage as opposed to other lineages of blood cells such as the myeloid lineage and the erythroid lineage.
Nomenclature, the system of naming things properly, is not trivial in this case because although lymphocytes are found in the bloodstream and originate in the bone marrow, they principally belong to the separate lymphatic system which interacts with the blood circulation.
Lymphopoiesis is now usually used interchangeably with the term "lymphocytopoiesis" - the making of lymphocytes - but other sources may distinguish between the two, stating that "lymphopoiesis" additionally refers to creating lymphatic tissue, while "lymphocytopoiesis" refers only to the creation of cells in that tissue. It is rare now for lymphopoiesis to refer to the creation of lymphatic tissues.
Myelopoiesis refers to 'generation of cells of the myeloid lineage' and erythropoiesis refers to 'generation of cells of the erythroid lineage' etc., so parallel usage has evolved in which lymphopoiesis refers to 'generation of cells of the lymphoid lineage'.
Observations on research going back well over 100 years had elucidated the two great classes of WBC - Myeloid and Lymphoid - and great advances in medicine and science have resulted from these studies. It was only natural to ask where these two great classes of cells arose, and after much work two cell types with some strong stem cell properties were isolated and defined - CMP, the common myeloid progenitor and CLP, the common lymphoid progenitor for mice.[2] But science is an additive game and it was eventually found these progenitors were not unique, and further that the two great families of Myeloid and Lymphoid were not disjoint, but rather two partially interwoven family trees. This is more than just nomenclature, it is new science that provides challenges of complexity yet offers new vistas of bio-science and the promise of early enhancement of private and public health issues. And it gives insight into the nature of redundancy and overlap in the immune system and hints how to use this to advantage.
Purpose
The complete loss of or loss of function of any WBC cell type is a serious health matter, but lymphopoiesis is absolutely necessary for life. Mature lymphocytes are a critical part of the immune system that (with the exception of memory B and T cells) have short lives measured in days or weeks and must be continuously generated throughout life by cell division and differentiation from cells such as common lymphoid progenitors (CLPs) in mice. Were this system to fail, the body would be largely undefended from infection.
The set comprising CLP cells and similar progenitors are themselves descendants of the pluripotential hemopoietic stem cell (pHSC) which is capable of generating all of the cell types of the complete blood cell system.[3] Despite their remarkable ability to generate the complete suite of lymphocytes, most progenitors are not true stem cells, however, and must be continually renewed by differentiation from the pHSC stem cell.[4]
Many progenitor cells are also referred to as transit cells,[5][6] sometimes also called transit amplifying cells, the meaning of this term being that the transit cell may found a new sub-lineage but the number of resultant cells is strictly limited (although possibly very large, even trillions yet finite) and the lineage is terminated by cells that die off (by apoptosis) or remain as cells that can no longer divide. Examples of such cells are CFUs (Colony-forming units - referred to as such because of their ability to form colonies in vitro in artificial media) such as CFU-T.[7]
In mice, transplantation of a single pHSC cell can reconstitute a sub-lethally irradiated host (i.e. a mouse that has been irradiated so that all leukocytes are killed) with all these lineages of cells, including all types of lymphocytes via CLPs. This has been known for more than 40 years.
Lymphopoiesis continues throughout life and so progenitor cells and their parent stem cells must always be present.[8]
Overview
In the case of mammals such as man, lymphopoiesis begins with limited passive provision by the mother of lymphocytes and substantial immunoglobulin G that cross the placenta and enter the fetus to provide some protection against pathogens, and also leukocytes that come from breast milk and enter circulation via the digestive tract.[9] It is often not effective in preventing infections in the newborn.[10]
However early in gestation the developing embryo has begun its own lymphopoiesis from the fetal liver. Lymphopoiesis also arises from the yolk sac.[11] This is in contrast to the adult where all lymphocytes originate in the bone marrow.[12]
There are four major types of lymphocytes, many sub-types, and hundreds or thousands of lymphocyte cell types that have been identified by scientists. All are generated by normal or abnormal lymphopoiesis except for certain artificial strains created in the laboratory by development from existing strains. Although lymphocytes are usually considered mature (as seen in blood tests) they are certainly not inert but can and do get around the body to anywhere there is a need; and when such need arises, new rounds of 'downstream' lymphopoiesis such as cell multiplication and differentiation may arise, coupled with intense mitotic and metabolic activity.
This is hardly a simple topic. In his 1976 text Immunology, Aging and Cancer immunologist and Nobel Prize winner Sir Frank Macfarlane Burnet speculated that the immune system might one day be found to be as complex as the nervous system. As the production of lymphocytes is so close to the central role of the immune response it is wise to approach the study of it with some humility in the face of the task, although there are general principles that help in understanding.
Process
| Lymphopoiesis Acronyms |
|---|
| • B-NK Progenitor for B and NK |
| • CB Cord blood |
| • CFU Colony-forming Unit |
| • CLP Common Lymphoid Progenitor |
| • CMP Common Myeloid Progenitor |
| • DC Dendritic Cell (Myeloid or Lymphoid) |
| • ELP Early Lymphoid Progenitors |
| • ETP the most primitive cells in the thymus are the Early Thymocyte Progenitors |
| • G-CSF Granulocyte Colony Stimulating Factor |
| • GM-CSF Granulocyte Macrophage Colony Stimulating Factor |
| • GMP Granulocyte Macrophage Progenitor; |
| • HSC pluripotential Hemopoietic Stem Cell |
| • MDC combined Macrophage and DC progenitor potential |
| • MEP megakaryocytic and erythroid progenitor |
| • MLP Multi-lymphoid Progenitor potential, any progenitor minimally able to give rise to B cells, T cells and NK cells |
| • MPP Multipotent Progenitor |
| • Notch Notch signaling pathway re T Cell commitment from progenitors |
Lymphopoiesis can be viewed in a mathematical sense as a recursive process of cell division and also as a process of differentiation, measured by changes to the properties of cells.
- Given that lymphocytes arise from specific types of limited stem cells - which we can call P (for Progenitor) cells - such cells can divide in several ways. These are general principles of limited stem cells.[13]
Considering the P as the ‘mother’ cell, but not a true stem cell, it may divide into two new cells, which are themselves identical, but differ to some degree from the mother. Or the mother cell P may divide unequally into two new daughter cells both of which differ from each other and also from the mother.
Any daughter cell will usually have new specialized abilities and if it is able to divide it will form a new sub-lineage. The difference of a daughter cell from the mother may be great, but it could also be much less, even subtle. What the P mother cell does not do is divide into two new P mother cells or a mother and a daughter; this is a matter of observation as such limited progenitor cells are known to not self-renew.
- There is a sort of exception when daughter cells at some level of the lineage may divide several times to form more seemingly identical cells, but then further differentiation and division will inevitably occur, until a final stage is reached in which no further division can occur and the cell type lineage is finally mature. An example of maturity is a plasma cell, from the B cell lineage, which produces copious antibody, but cannot divide and eventually dies after a few days or weeks.
- The progenitor CLP of the mouse or the progenitor MLP of the human differentiates into lymphocytes by first becoming a lymphoblast (Medical Immunology, p. 10). It then divides several more times to become a prolymphocyte that has specific cell-surface markers unique to either a (1) T cell or (2) B cell. The progenitor can also differentiate into (3) natural killer cells (NK) and (4) dendritic cells.
- T Cells, B Cells and NK Cells (and all other Innate lymphoid cells) are unique to the lymphocyte family, but dendritic cells are not. Dendritic cells of identical appearance but different markers are spread throughout the body, and come from either lymphoid and myeloid lineages, but these cells may have somewhat different tasks and may take up lodging preferentially in different locations.[14] (Revise in light of new research) This is now an open question; also, the different dendritic cell lineages may have different ‘tasks’ and stay in different ‘locations.’ [15]
T and B lymphocytes are indistinguishable histologically(that is, under a light microscope they cannot be told apart.)Indeed, the inactive B and T cells are so featureless with few cytoplasmic organelles and mostly inactive chromatin that until the 1960s textbooks could describe these cells, now the central focus of immunology, as having no known function!![16]
However T and B lymphocytes are very distinct cell lineages and they ‘grow up’ in different places in the body. They perform quite different (although co-operative) functions in the body. No evidence has ever been found that T and B cells can ever interconvert. T and B cells are biochemically distinct and this is reflected in the differing markers and receptors they possess on their cell surfaces. This seems to be true in all vertebrates, although there are many differences in the details between the species.
- Regardless of whether the CLP (mouse) or MLP or a small closely related set of progenitor cells take credit for generating the profusion of lymphocytes, it remains an interesting observation that the same lymphoid progenitors can still opt to generate some cells that are clearly identifiably myeloid.
Lymphopoiesis for T cells
T cells are formed in bone marrow then migrate to the cortex of the thymus to undergo maturation in an antigen-free environment for about one week where a mere 2-4% of the T cells succeed. The remaining 96-98% of T cells die by apoptosis and are phagocytosed by macrophages in the thymus. So many thymocytes (T cells) die during the maturation process because there is intensive screening to make sure each thymocyte has the ability to recognize self-peptide:self-MHC complex and for self tolerance. The apoptosed thymocyte dies and is quickly recycled.
Upon maturity, there are several forms of thymocytes including [17]
- T-helper (needed for activation of other cells such as B cells and macrophages),
- T-cytotoxic (which kill virally infected cells),
- T-memory (T cells that remember antigens previously encountered), and
- T-suppressor cells (which moderate the immune response of other leukocytes). Also called T-regulatory cells (Tregs)
When T-Cells become activated they undergo a further series of developments. A small, resting T lymphocyte rapidly undergoes blastogenic transformation into a large lymphocyte (13–15μm). This large lymphocyte (known in this context as a lymphoblast) then divides several times to produce an expanded population of medium (9–12μm) and small lymphocytes (5–8μm) with the same antigenic specificity.[18] Final activated and differentiated T lymphocytes are once again morphologically indistinguishable from a small, resting lymphocyte. Thus the following developmental states may be noticed in sequence in blood tests:
- Prolymphocyte
- Large lymphocyte
- Small lymphocyte
Basic Map of T Cell lymphopoiesis
This basic map of T Cell formation, in sequence, is simplified and is akin to textbook descriptions, and may not reflect latest research.(Medical Immunology, p. 119)
In the thymus
- MLP
- ETP
- DN1
- (B; Mφ)
- DN2
- (DC; NK)
- DN3
- (γδ)
- DN4
- DP
- (TNK; CD4; CD8; Treg)
In the Periphery
- (Th1; Th2)
T cell development
Unlike other lymphoid lineages, T cell development occurs almost exclusively in the thymus. T-lymphopoiesis does not occur automatically but requires signals generated from the thymic stromal cells. The process has an astonishingly complex beauty to it. Several stages at which specific regulators and growth factors are required for T cell development to proceed have been defined. Interestingly, later in T cell development and its maturation these same regulatory factors again are used to influence T cell specialization.
T cells are unique among the lymphocyte populations in their ability to further specialize as mature cells and become yet more mature. And T Cells come in many flavors, for example: the conventional TcRαβ T cells; the so-called unconventional TcRγδ T cells; NKT cells; and T regulatory cells (Treg). Details regarding the developmental and life cycle of the unconventional T cells are less well-described compared to the conventional T cells.
Stages of T cell maturation
Stage One: Thymic Migration
Multipotent lymphoid progenitors (MLP) enter the T cell pathway as they immigrate to the thymus. The most primitive cells in the thymus are the early thymocyte progenitors (ETP), which retain all lymphoid and myeloid potential but exist only transiently, rapidly differentiating into T and NK lineages. (Medical Immunology, p. 118)
Stage Two: Proliferative Expansion and T Lineage Commitment
Final commitment to the T cell lineage occurs within the thymic microenvironment, the microscopic structures of the thymus where T cells are nurtured. The most primitive T cells retain pluripotential ability and can differentiate into cells of the myeloid or lymphoid lineages (B cells, DC, T cells, or NK cells).
More differentiated double negative T cells (DN2 cells) have more limited potentiality but are not yet fully restricted to the T cell lineage (they can still develop into DC, T cells, or NK cells). Later on, they are fully committed to the T cell lineage- when thymoctyes expressing Notch1 receptors engage thymic stromal cells expressing Notch1 ligands, the thymocytes become finally committed to the T-cell lineage. See Gallery Image "Double Negatives"
With the commitment to the T cell lineage, begins a very complex process known as TcR gene rearrangement. This creates an enormous diversity of T cells bearing antigen receptors. Afterward some T cells leave the thymus to migrate to the skin and mucosae.
Stage Three: β-Selection
Stage Four: T Cell Receptors Selection
Only 2% to 3% of the differentiating thymocytes, those that express TcR capable of interaction with MHC molecules, but tolerant to self-peptides, survive the Stage Four selection process.
Stage Five: Continuing Differentiation in the Periphery
It was previously believed that the human thymus remained active as the site of T cell differentiation only until early adulthood and that later in adult life the thymus atrophies, perhaps even vanishing. Recent reports indicate that the human thymus is active throughout adult life. Thus several factors may contribute to the supply of T cells in adult life: generation in the thymus, extra-thymic differentiation, and the fact that memory T cells are long-lived and survive for decades.
T cell types
- Unconventional T cells
The thymus also gives rise to the so-called ‘unconventional T cells’ such at γδ T cells, Natural Killer T cells (NKT) and regulatory T cells (Treg).
- γδ T cells
γδT cells represent only 1% to 5% of the circulating T cells, but are abundant in the mucosal immune system and the skin, where they represent the dominant T cell population. These ‘non-MHC restricted T cells’ are involved in specific primary immune responses, tumor surveillance, immune regulation and wound healing.
Several differences between αβ and γδ T cell development have been described. They emigrate from the thymus in "waves" of clonal populations, which home to discrete tissues. For example, one kind is found in the peripheral blood while another predominates in the intestinal tract.
- Natural Killer T cells
Human NKT cells are a unique population and are thought to play an important role in tumor immunity[19] and immunoregulation.
- T Regulatory cells
"Tregs" are considered as naturally occurring regulatory T cells. Tregs comprised about 5% of the circulating CD4+ T cells. These cells are thought to possess important an autoimmunity property by regulating 'autoreactive' T cells in the periphery. (Medical Immunology, p. 117-122)
Lymphopoiesis for B cells
B cells are formed and mature in bone marrow (and spleen).
It is a good mnemonic aide that B cells are formed in the bone marrow, but it is a mere coincidence since B cells were first studied in the chicken's bursa of Fabricius and it is from this bursa that B cells get their name.
These B cells then leave the bone marrow and migrate to peripheral lymphoid tissues, such as a lymph node. Once in a secondary lymphoid organ the B cell can be introduced to an antigen that it is able to recognize.
Through this antigen recognition and other cell interactions the B cell becomes activated and then divides and differentiates to become a plasma cell. The plasma cell, a B cell end product, is a very active antibody-secreting cell that helps protect the body by attacking and binding to antigen.
Even after many decades of research, some controversy remains as to where B cells mature and 'complete their education', with the possibility remaining that the site may also partially be peri-intestinal lymphoid tissues.[20]
B lymphopoiesis occurs exclusively in the bone marrow and B lymphocytes are made continuously throughout life there in a 'microenvironment' composed of stromal cells, extracellular matrix, cytokines and growth factors, which are critical for proliferation, differentiation, and survival of early lymphocyte and B-lineage precursors.
The relative proportion of precursor B cells in the bone marrow remains rather constant throughout the life span of an organism. There are stages such as Pre-B-I cells (5% to 10% of the total); Pre-B-II cells (60% to 70%) while the remaining 20% to 25% are immature B cells. Most textbooks say that B Cells mature in the bone marrow but, generally, immature B cells migrate to the spleen for 'higher education' of some sort where they go through transitional stages before final maturation.(Medical Immunology, p. 136)
B lymphocytes are identified by the presence of soluble immunoglobulin G (IgG). This is the most common protective immunoglobulin in the adult body. After antigenic stimulation, B cells differentiate into plasma cells that secrete large quantities of soluble IgG. This is the final stage of B lymphopoiesis but it is the clincher because the plasma cells must either issue antibody close to a source of infection, or disseminate it in the blood to fight an infection at a distance or in an inaccessible part of the body.
Basic map of B cell lymphopoiesis
A generally regarded valid map of B cell lymphopoiesis is as follows in sequence, in two parts with the first being in the bone marrow and the second in the spleen:.[21] The development process in the bone marrow occurs in Germinal Centers.
In the bone marrow
- Pro-B
- Pre-B-I
- Pre-B-II large
- Pre-B-II small
- Imm(ature)
In the spleen
- T1
- T2/T3
- (Marginal Zone (MZ); B-1 ; B-2)
- B-2 further differentiate into:
- (Germinal Center (GC); Memory ; Plasma )
Lymphopoiesis for NK cells
NK cells, which lack antigen specific receptors, develop in the bone marrow. After maturation and release from the marrow they circulate in the blood through their lifetime seeking opportunity. The opportunity they seek is to encounter and recognize and then kill abnormal cells such as cancer or virally infected cells. It is well known that lymphocytes never have granules or at least not granules that are readily visible even upon staining. However NK cells are the exception. They do have numerous granules which provide their ability to kill cells and these granules are why NK cells have an alternate name, LGL, Large Granular Lymphocytes.
NK cells not only have a catchy movie-title name (Natural Killer) but are also the only lymphocytes considered part of the innate immune system (in contrast to the adaptive immune system. Yet they are much more closely related to T cells (part of the adaptive immune system) than to other cells of the innate immune system. NK cells not only share many surface markers, functions and activities in common with T Cells, they also arise from a common T/NK progenitor. The T/NK precursor is also believed to be the source of a subpopulation of lymphoid DC. (Medical Immunology, p. 121)
NK cells have a definition 'barcode' as CD3, CD16+, CD56t lymphocytes. (See Barcode Section of this article). NK progenitors can be found mainly in the thymus (mouse), but the thymus is not absolutely required for NK development. Probably NK cells can develop in a variety of organs but the major site of NK cell development is not known.
In humans, the majority (85–90%) of the NK cells have a high cytolytic capacity (the ability to lyse cells). A smaller subset (10–15%) called NK 'CD56 bright' is chiefly responsible for cytokine production and has enhanced survival. Traveling to lymph nodes the 'CD56 bright' NK cells differentiate again into mature NK cells which express killer cell immunoglobulin-like receptors (KIR), natural cytotoxicity receptors (NCR), and critical adhesion molecules. (Medical Immunology, p. 122)
Lymphopoiesis for dendritic cells
Dendritic cell is usually abbreviated DC or DCs. The process by which CLP cells may differentiate to generate dendritic cells of lymphoid lineage is not yet well defined.[22]
DCs are highly specialized and efficient antigen-presenting cells. Cells identical in appearance come both from a myeloid lineage (referred to as myeloid dendritic cells) and also from a lymphoid lineage (referred to as plasmacytoid dendritic cells).
The development and regulation of DC is not well-characterized. While the DC precursors have been identified in the human fetal liver, thymus, and bone marrow, during adult life DC are thought to be produced only from the bone marrow and released into the blood to wander and settle down. Overall a large number of DC of varying types are dispatched throughout the body, especially at epithelia such as skin, to monitor invaders and nibble their antigens. (Medical Immunology, p. 122)
Comparison of killers from lymphopoiesis
Lymphocytes have a number of alarming properties such as the ability to wander around the body and take up lodging almost anywhere, and while on the way issue commands in the form of cytokines and chemokines and lymphokines, commands that affect many cell types in the body and which may also recursively induce further lymphopoiesis. One strong behavior pattern that captivates researchers and the public alike is the ability of lymphocytes to act as police, judge and executioner to kill other cells or demand that they suicide, a command that is usually obeyed. There seems to be no other sentencing option available.
Killers are distinguished from cells such as macrophages that eat other cells or munch debris by a method called phagocytosis. Killers do not use phagocytosis, they just kill and leave the clean-up to other cells.
Killers are known to attack virus-infected cells and cells that have become cancerous. Because of these abilities much research has been done into transforming these qualities into medical therapy but progress has been slow.
Here is the parade of killers and how they work:
- Cytotoxic T cells
(also called Tc or antigen-specific cytolytic T lymphocytes (CTL)).[23] Tc kill by apoptosis and either splash their target with perforin or granzymes or else use Fas-Fasl Interaction to command target elimination. This kills cells that are infected and display antigen.
- NK cells (also called LGL (large granular lymphocytes))
These kill with exactly the same methods as Tc, but have no interaction with any antigen. They select their targets based on typical molecules displayed by cells that are under stress by viral infection. NK Cells mainly are in the circulation (5-15% of the circulating lymphocytes) yet are also distributed in tissues everywhere.[24]
- LAK cells (Lymphokine-activated killer) are a laboratory/clinical subset of NK Cells promoted by IL-2 to attack tumor cells.[25]
- NKT cells see Natural Killer T cell main article
Natural Killer T Cells. Human NK T cells are a unique population (which express NK cell markers such as CD56 and KIR). NKT cells are thought to play an important role in tumor immunity and immunoregulation. (Medical Immunology, p. 135), yet little is known. Recent evidence suggests a role working together with hepatic stellate cells being a liver-resident antigen-presenting cell that presents lipid antigens to and stimulates proliferation of NKT cells.
- Natural killer-like T cells
A heterogeneous group with ill-defined properties.
However in summary there is no known cell or set of cells that is capable of killing cancerous cells in general.
Labeling lymphopoiesis
Because all WBCs are microscopic, colorless and often seemingly identical in appearance they are individually identified by their natural chemical markers, many of which have been analyzed and named. When two cells have the same markers, the reasonable assumption is made that the cells are identical at that time. A set of markers is colloquially describes as the barcode for that cell or that cell line.
- Here is an example of how a barcode can come to be, for the all-important HSC as an example.
HSC are technically described as: lacking FMS-like tyrosine kinase 3 (Flt3) and lacking the markers specific to discrete lymphoid lineages (Lin), but expressing high levels of Sca1 and c-kit; HSC also express CD44, low levels of Thy1.1 (CD90), but no IL-7Ra or CD27.
This is called the (surface) phenotype of an HSC. It can be expressed as a set (Lin2, Sca1high, c-kit high, CD44+, Thy1.1low, CD27 2, and IL-7Ra2). This set is a ‘barcode’ for the HSC, akin to the barcode label attached to your chicken-wing plastic bag for checkout at a supermarket! Scientists use these barcodes to check, categorize and accumulate cells for many purposes often using laboratory methods such as cell flow cytometry. These barcodes partially define the modern meaning of phenotype for leukocytes.
Progression of HSC differentiation and lineage commitment is indicated by changes in this phenotype. That is, as the cell changes, the markers will also change and the barcode will change.
- Typical barcodes for some cell types appearing in this article.
| Cell Type | Barcode |
|---|---|
| ETP | C-Kit+, CD44+, CD25- |
| DN1 | CD44+, CD25- |
| DN2 | CD44+, CD25+ |
- Note explaining the barcode parameter details: Flt3 is a cytokine tyrosine kinase receptor thought to be important in early lymphoid development. In addition, Flt3 plays a major role in maintaining B lymphoid progenitors. CD27 plays a role in lymphoid proliferation, differentiation, and apoptosis. The acquisition of CD27 and Flt3 by the HSC coincides with the loss of long-term repopulating potential. At this stage the cells retain both lymphoid and myeloid potential and are referred to as multipotent progenitors. (Medical Immunology, p. 114)
Knowledge development regarding lymphopoiesis
New questions emerge in immunology continuously as though there were a stem cell for questions. For example it was thought that the process of lymphopoiesis was a direct, orderly unidirectional sequence. But it is not clear if end-stage lymphocytes come from progenitors that are homogeneous populations or overlapping populations. Nor is it clear whether lineages of lymphocytes develop via a continuum of differentiation with a progressive loss of lineage options or whether abrupt events result in the acquisition of certain properties.[26]
Changes in cytoplasm, morphology of the cell nucleus, granules, cell internal biochemistry, signaling molecules and cell surface markers are difficult to correlate with definite stages in lymphopoiesis. The morphological differences do not just correspond to steps in mitosis (somatic cell division), but result from continuous "maturation processes"of the cell nucleus as well as of the cytoplasm and so one must not be too rigid about morphological distinctions between certain cell stages.[27]
- Models and updates on the lymphopoiesis family tree
Until recently the model of the CMP generating all myeloid cell and the CLP generating all lymphoid cells was considered necessary and sufficient to explain the known facts observed in the generation of WBCs, and it is still found in most basic textbooks. However beginning around 2000 and gaining momentum after 2005[28] in both studies on man and mouse, new complexities were noted and published in papers. These studies are important now mainly to immunology researchers but are likely to eventually lead to changes in medical treatments.
The changes were sparked by observations that lymphopoiesis did not always break into two lineages at the level of the CLP. Worse, some macrophages (long considered a myeloid lineage) could be generated by lymphoid lineage progenitors. In essence focus has been shifted away from the CLP to the MLP (lymphoid-specified progenitors), which are clearly lymphoid progenitors yet retain some myeloid potential, particularly the interesting ability in both man and mouse to make macrophages - one of the most versatile of immune cell defenders - and also many dendritic cells, the best 'watchdogs' of antigen invaders.
However, whatever the details may turn out to be, the process of lymphopoiesis always seems to relentlessly give rise to progeny with special attributes and abilities - "superpowers" so to speak - but with progressively more restricted lymphoid developmental potential.
Stages of development
The old model: lymphoid vs myeloid
This model of lymphopoiesis had the virtue of relative simplicity, agreement with nomenclature and terminology, and is still essentially valid for the laboratory mouse.
- pHSC pluripotent, self-renewing, hematopoietic stem cells [29] which give rise to
- MPP multipotent progenitors, which give rise to
- ELP (or PRO) Prolymphocytes, early lymphoid progenitors, and finally to the
- CLP Common lymphoid progenitor, a cell type fully committed to the lymphoid lineage.
pHSC, MPP and ELP cells are not fully committed to the lymphoid lineage because if one is removed to a different location it may differentiate into non-lymphoid progeny. However CLP are committed to the lymphoid lineage. The CLP is the transit cell responsible for these (generally parallel) stages of development, below:
- NK cells
- Dendritic cells (lymphoid lineage; DC2 [30])
- Progenitor B cells
- Pro-B cells => Early Pro (or pre-pre)-B cells => Late Pro (or pre-pre)-B cells
- Large Pre-B cells => Small Pre-B cells
- Immature B cells
- B Cells => (B1 cells; B2 cells)
- Plasma cells
- Pro-T cells
- T-cells
New model: Mixed myeloid/lymphoid model
Research on new models (not mice)
By 2008 it was found that "the majority of early thymic progenitor cells do not commit to becoming T cells by the time they get to the thymus gland. ETP cells retained the ability to become either T cells or myeloid cells. "[31] [32]
See also :[33] [34] [35] [36] [37] [38]
Graphical view of the old model vs mixed myelo-lymphoid model
-
_Grayscale.jpg)
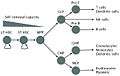
Side by side. Comparing the new and old lineage models.
-
Revised Lineage Myelo-lymphoid flowchart.
General immunology reference texts
Texts in bold are the most heavily cited in this article.
- Cell Communication in Nervous and Immune System; Gundelfinger, Seidenbecher, Schraven; Springer Berlin Heidelberg New York; 2006; ISBN 3-540-36828-0
- Color Atlas of Hematology; Theml et al.; Thieme; 2004; ISBN 1-58890-193-9
- Dynamics of Cancer; Steven A. Frank; Princeton University Press, Princeton, New Jersey; 2007; ISBN 978-0-691-13366-9, Creative Commons Public License
- Fundamental Immunology, 5th edition; William E. Paul (Editor); Lippincott Williams & Wilkins Publishers; 2003; ISBN 0-7817-3514-9
- Immuno-Biology: The Immune System in Health and Science, 6th Edition; Janeway, Travers; 2005; Garland Science Publishing, New York; ISBN 0-8153-4101-6
- Immunology Introductory Textbook (ebook;revised 2nd edition); Nandini Shetty; New Age International (P) Limited, Publishers, India; 2005; ISBN 81-224-2335-3
- Instant Notes in Immunology, 2nd ed.; Lydyard, Whelan, Fanger; Taylor and Francis Group; 2004; China Version ISBN 978-7-03-025225-8; 46RMB Wangfujing Bookstore
- Medical Immunology—6th ed.; G. Virella, Editor; Informa Healthcare USA, Inc; 2007; ISBN 978-0-8493-9696-0
- Stem Cell Biology; Marshak, Gardner, Gottlieb; Cold Spring Harbor Laboratory Press; 2001; ISBN 0-87969-575-7/01
- Textbook of Human Development and Histology; Zhong Cuiping et al.; Shanghai Scientific and Technical Publishers; 2006; ISBN 7-5323-8230-3
- Textbook of Medical Immunology (Immunology, 7th Edition); LIM Pak Leong; Elsevier (Singapore) Pte Ltd.; 2006; ISBN 0-323-03399-7
References
- ↑ Birbrair, Alexander; Frenette, Paul S. (2016-03-01). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences: n/a–n/a. doi:10.1111/nyas.13016. ISSN 1749-6632.
- ↑ Birbrair, Alexander; Frenette, Paul S. (2016-03-01). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences: n/a–n/a. doi:10.1111/nyas.13016. ISSN 1749-6632.
- ↑ Stem Cell Biology, page 307
- ↑ Birbrair, Alexander; Frenette, Paul S. (2016-03-01). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences: n/a–n/a. doi:10.1111/nyas.13016. ISSN 1749-6632.
- ↑ Dynamics of Cancer, page 251
- ↑ transit cells - q.v., Also called
- ↑ CFU-T
- ↑ Birbrair, Alexander; Frenette, Paul S. (2016-03-01). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences: n/a–n/a. doi:10.1111/nyas.13016. ISSN 1749-6632.
- ↑ Transfer of maternal colostral leukocytes promotes development of the neonatal immune system
- ↑ Pryhuber, Gloria S. (2015). "Postnatal Infections and Immunology Affecting Chronic Lung Disease of Prematurity". Clinics in Perinatology. 42 (4): 697–718. doi:10.1016/j.clp.2015.08.002. ISSN 0095-5108; Access provided by the University of Pittsburgh
- ↑ Adult T-cell Progenitors Retain Myeloid Potential; Nature; 2008
- ↑ Textbook of Human Development and Histology, p.176
- ↑ Birbrair, Alexander; Frenette, Paul S. (2016-03-01). "Niche heterogeneity in the bone marrow". Annals of the New York Academy of Sciences: n/a–n/a. doi:10.1111/nyas.13016. ISSN 1749-6632.
- ↑ Immuno-Biology, The Immune System in Health and Science.
- ↑ Fundamental Immunology 5th edition
- ↑ Immuno-Biology, The Immune System in Health and Science. Garland Science Publishing
- ↑ Textbook of Medical Immunology, page 5
- ↑ Medical Immunology, page 23
- ↑ Tumor Immunity and Cancer Immunotherapy
- ↑ Medical Immunology, page 22
- ↑ Medical Immunology, p. 123
- ↑ Fundamental Immunology; Paul; Ch. 15 "DISTRIBUTION OF DENDRITIC CELLS IN VIVO: A MULTIMEMBER FAMILY"
- ↑ Immunology; Lydyard et al; p. 22, 132-137
- ↑ Immunology; Lydyard et al; p. 15, 18-20,41
- ↑ Immunology; Lydyard et al; p. 20, 259-260
- ↑ Medical Immunology; Litwin, p. 122
- ↑ Color Atlas of Hematology 2004
- ↑ "The Earliest Thymic Progenitors for T cells Possess Myeloid Lineage Potential"; Bell, Bhandoola; Vol 452, 10 April 2008, doi:10.1038/nature06840
- ↑ Medical Immunology, Litwin, p.115
- ↑ Textbook of Medical Immunology, page 31
- ↑ Research Findings May Shed Light on T-cell Leukemias and Immunodeficiencies. Bhandoola. April 9, 2008;
- ↑ Blood Lines Redrawn; Thomas Graf; Nature Vol 452 10 April 2008 p.702-703
- ↑ The Common Myelolymphoid Progenitor: A Key Intermediate Stage in Hemopoiesis Generating T and B Cells; Min Lu, Hiroshi Kawamoto, Yoshihiro Katsube, Tomokatsu Ikawa, and Yoshimoto Katsura; J. Immunol. 2002;169;3519-3525
- ↑ Identification of Flt3 + Lympho-Myeloid Stem Cells Lacking Erythro-Megakaryocytic Potential: A Revised Road Map for Adult Blood Lineage Commitment; Lund Strategic Research Center for Stem Cell Biology; Cell; Vol. 121, 295–306, April 22, 2005
- ↑ Adult T-cell progenitors retain myeloid potential; Haruka Wada, Kyoko Masuda, Rumi Satoh , Kiyokazu Kakugawa, Tomokatsu Ikawa, Yoshimoto Katsura & Hiroshi Kawamoto; Nature Vol 452 10 April 2008
- ↑ The earliest thymic progenitors for T cells possess myeloid lineage potential; J. Jeremiah Bell, Avinash Bhandoola; Nature; Vol 452, 10 April 2008, p. 764-768
- ↑ Revised map of the human progenitor hierarchy shows the origin of macrophages and dendritic cells in early lymphoid development; Dick et al; Nature Immunology; Volume 11 Number 7 July 2010 p. 585-595
- ↑ Not a split decision for human hematopoiesis; Kenneth Dorshkind; Nature Immunology Volume 11 Number 7 July 2010 p. 569-570
Additional images
Alternate views of lineages
-
Blood cell lineage. For scale, note that megakaryocytes (50-100 μm) are 10 to 15 times larger than a typical red blood cell.
-
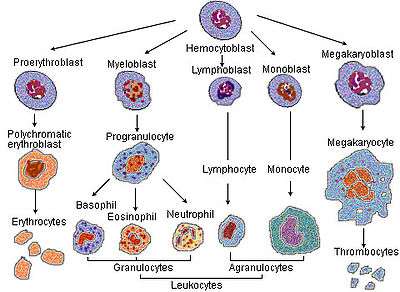
Blood cell lineage. Based on self-renewal ability.
-
_diagram.png)
Schematic view. Well-defined lineages.
-
_Grayscale.jpg)
Side by side. Comparing the new and old lineage models.
External links
- The www.copewithcytokines.de Mini-portal to Lymphopoiesis terminology
- Lymphopoiesis at the US National Library of Medicine Medical Subject Headings (MeSH)
- "Lymphopoiesis" at Dorland's Medical Dictionary