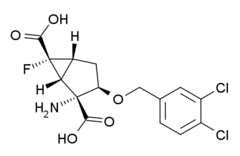
MGS-0039
 | |
| Clinical data | |
|---|---|
| ATC code | None |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 569686-87-9 |
| PubChem (CID) | 9886034 |
| IUPHAR/BPS | 1397 |
| ChemSpider | 8061707 |
| Chemical and physical data | |
| Formula | C15H14Cl2NO5 |
| Molar mass | 378.179 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
MGS-0039 is a drug that is used in neuroscientific research, which acts as a potent and selective antagonist for group II of the metabotropic glutamate receptors (mGluR2/3).[1][2] It produces antidepressant and anxiolytic effects in animal studies,[3][4][5][6] and has been shown to boost release of dopamine and serotonin in specific brain areas.[7][8] Research has suggested this may occur through a similar mechanism as that suggested for the similarly glutaminergic drug ketamine.[9][10]
References
- ↑ Chaki S, Yoshikawa R, Hirota S, Shimazaki T, Maeda M, Kawashima N, Yoshimizu T, Yasuhara A, Sakagami K, Okuyama S, Nakanishi S, Nakazato A. MGS0039: a potent and selective group II metabotropic glutamate receptor antagonist with antidepressant-like activity. Neuropharmacology. 2004 Mar;46(4):457-67. PMID 14975669
- ↑ Nakazato A, Sakagami K, Yasuhara A, Ohta H, Yoshikawa R, Itoh M, Nakamura M, Chaki S. Synthesis, in vitro pharmacology, structure-activity relationships, and pharmacokinetics of 3-alkoxy-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid derivatives as potent and selective group II metabotropic glutamate receptor antagonists. Journal of Medicinal Chemistry. 2004 Aug 26;47(18):4570-87. PMID 15317467
- ↑ Shimazaki T, Iijima M, Chaki S. Anxiolytic-like activity of MGS0039, a potent group II metabotropic glutamate receptor antagonist, in a marble-burying behavior test. European Journal of Pharmacology. 2004 Oct 6;501(1-3):121-5. PMID 15464070
- ↑ Yasuhara A, Nakamura M, Sakagami K, Shimazaki T, Yoshikawa R, Chaki S, Ohta H, Nakazato A. Prodrugs of 3-(3,4-dichlorobenzyloxy)-2-amino-6-fluorobicyclo[3.1.0]hexane-2,6-dicarboxylic acid (MGS0039): a potent and orally active group II mGluR antagonist with antidepressant-like potential. Bioorganic and Medicinal Chemistry. 2006 Jun 15;14(12):4193-207. PMID 16487713
- ↑ Yoshimizu T, Shimazaki T, Ito A, Chaki S. An mGluR2/3 antagonist, MGS0039, exerts antidepressant and anxiolytic effects in behavioral models in rats. Psychopharmacology (Berlin). 2006 Jul;186(4):587-93. PMID 16612616
- ↑ Stachowicz K, Wierońska J, Domin H, Chaki S, Pilc A. Anxiolytic-like activity of MGS0039, a selective group II mGlu receptor antagonist, is serotonin- and GABA-dependent. Pharmacological Reports. 2011;63(4):880-7. PMID 22001975
- ↑ Kawashima N, Karasawa J, Shimazaki T, Chaki S, Okuyama S, Yasuhara A, Nakazato A. Neuropharmacological profiles of antagonists of group II metabotropic glutamate receptors. Neuroscience Letters. 2005 Apr 22;378(3):131-4. PMID 15781145
- ↑ Karasawa J, Yoshimizu T, Chaki S. A metabotropic glutamate 2/3 receptor antagonist, MGS0039, increases extracellular dopamine levels in the nucleus accumbens shell. Neuroscience Letters. 2006 Jan 30;393(2-3):127-30. PMID 16233956
- ↑ Pałucha-Poniewiera A, Wierońska JM, Brański P, Stachowicz K, Chaki S, Pilc A. On the mechanism of the antidepressant-like action of group II mGlu receptor antagonist, MGS0039. Psychopharmacology (Berlin). 2010 Dec;212(4):523-35. PMID 20703449
- ↑ Koike H, Iijima M, Chaki S. Involvement of the mammalian target of rapamycin signaling in the antidepressant-like effect of group II metabotropic glutamate receptor antagonists. Neuropharmacology. 2011 Dec;61(8):1419-23. PMID 21903115
This article is issued from Wikipedia - version of the 4/2/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.