Methylenetriphenylphosphorane
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Methylene(triphenyl)phosphorane | |
| Identifiers | |
| 3487-44-3 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 121606 |
| PubChem | 137960 |
| |
| |
| Properties | |
| C19H17P | |
| Appearance | yellow solid |
| Density | 1.19 g/cm3 |
| decompose | |
| Solubility | THF |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Methylenetriphenylphosphorane is an organophosphorus compound with the formula Ph3PCH2. It is the parent member of the phosphorus ylides, popularly known as Wittig reagents. It is a highly polar, highly basic species.
Preparation and use
Methylenetriphenylphosphorane is prepared from triphenylphosphine and methyl bromide followed by deprotonation of the resulting phosphonium salt using a strong base like butyllithium:[1]
- Ph3PCH3Br + BuLi → Ph3PCH2 + LiBr + BuH
The compound is generally not isolated, instead it is used in situ.
Methylenetriphenylphosphorane is used to replace oxygen centres in aldehydes and ketones with a methylene group:
- R2CO + Ph3PCH2 → R2C=CH2 + Ph3PO
The phosphorus-containing product is triphenylphosphine oxide.
Structure
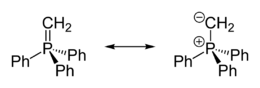
Crystallographic characterization of the colourless ylide reveals that the phosphorus is approximately tetrahedral. The PCH2 centre is planar and the P=CH2 distance is 1.661 Å, which is much shorter than the P-Ph distances (1.823 Å).[2] The compound is usually described as a combination of two resonance structures:
- Ph3P+CH2− ↔ Ph3P=CH2
References
- ↑ Georg Wittig U. Schoellkopf (1973). "Methylenecyclohexane". Org. Synth.; Coll. Vol., 5, p. 751
- ↑ Bart, J. C. J. "Structure of the non-stabilized phosphonium ylid methylenetriphenylphosphorane". Journal of the Chemical Society B: Physical Organic J. Chem. Soc. B. 1969: 350–365. doi:10.1039/J29690000350.