OSTE polymers
OSTE polymers is a novel polymer platform comprising Off-Stoichiometry Thiol-Enes (OSTE) and Off-stoichiometry thiol-ene-epoxies (OSTE+). The polymer resins were originally developed by Tommy Haraldsson and Fredrik Carlborg at the group of Micro and Nanosystems[1] at the Royal Institute of Technology (KTH) to bridge the gap between research prototyping and commercial production of microfluidics devices.[2] The resins were later adapted and improved for commercial applications by the Swedish start-up Mercene Labs AB[3] under the name OSTEMER.
The OSTE polymers comprise off-stoichiometry blends of thiols and allyls. After complete polymerization, typically by UV micromolding, the polymer articles contain a well-defined number of unreacted thiol or allyls groups both on the surface and in the bulk. These surface anchors can be used for subsequent direct surface modification or bonding.[4]
In later versions epoxy monomers were added to form ternary thiol-ene-epoxy monomer systems (OSTE+), where the epoxy in a second step reacts with the excess of thiols creating a final polymer article that is completely inert.[5] Some of the critical features of OSTE+ polymers include uncomplicated and rapid fabrication of complex structures in a standard chemistry labs, hydrophilic native surface properties and covalent bonding via latent epoxy chemistry.[6]
Reaction Mechanism
The OSTE resins are cured via a rapid thiol-ene “Click” reaction between thiols and allyls. The thiols and allyls react in a perfectly alternating fashion and has a very high conversion rate (up to 99%),[7] the initial off-stoichiometry of the monomers will exactly define the number off unreacted groups left after the polymerization. With the right choice of monomers very high off-stoichiometry ratios can be attained while maintaining good mechanical properties [8]
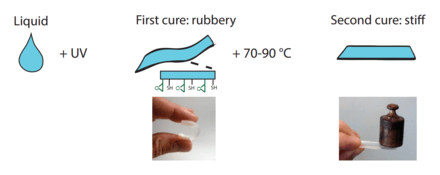
The off-stoichiometry thiol-ene-epoxies, or OSTE+ polymers, are created in a two-step curing process where a first rapid thiol-ene reaction defines the geometric shape of the polymer while leaving an excess of thiols and all the epoxy unreacted. In a second step all the remaining thiol groups and the epoxy groups are reacted to form an inert polymer. [Refer figure below] [9]
Properties
OSTE polymers
The main advantages put forward of the UV-cured OSTE polymers in microsystems have been their i) dry bonding capacity by reacting a polymer with thiol excess to a second polymer with allyl excess at room-temperature using only UV-light, ii) their well-defined and tunable number of surface anchors (thiols or allyls) present on the surface that can be used for direct surface modification[10] and iii) their wide tuning range of mechanical properties from rubbery to thermoplastic-like depending only on the choice of off-stoichiometry.[11][8] The glass transition temperature typically varies from below room-temperature for high off-stoichiometric ratios to 75 °C for a stoichiometric blend of tetrathiol and triallyl.[12] They are typically transparent in the visible range. A disadvantage put forward with the OSTE-polymers is the leaching out of unreacted monomers at very high off-stoichiometric ratios which may affect cells and proteins in lab-on-chips,[8] although cell viability has been observed for cell cultures on low off-stoichiometric OSTE.[13]
OSTE+ polymers
The dual-cure thiol-ene-epoxies, or OSTE+ polymers, differ from the OSTE-polymers in that they have two separated curing steps. After the first UV-initiated step, the polymer is rubbery and can easily be deformed[14] and it has surface anchors available for surface modification.[15] During the second step, when all the thiols and epoxies are reacted the polymer stiffens and can bond to a wide number of substrates, including itself, via the epoxy chemistry. The advantages put forward for the OSTE+ are i) their unique ability for integration and bonding via the latent epoxy chemistry and the low built-in stresses in the thiol-enes polymers[16] ii) their complete inertness after final cure iii) their good barrier properties[17] and the possibility to scale up manufacturing using industrial reaction injection molding.[18] Both stiff and rubbery versions of the OSTE+ polymers have been demonstrated, showing their potential in microsystems for valving and pumping similar to PDMS components, but with the benefit of withstanding higher pressures.[14] The commercial version of the OSTE+ polymer, OSTEMER 322, has been shown to be compatible with many cell lines.[19]
Fabrication
OSTE polymers
The OSTE resins can be cast and cured in a structured silicone molds[20] or coated permanent photoresist.[21] OSTE polymers have also shown excellent photostructuring capability[22] using photomasks, enabling for example powerful and flexible capillary pumps.[23]
OSTE+ polymers
The OSTE+ resins are first UV-cured in the same way as the OSTE-polymers but are later thermally cured to stiffen and bond to a substrate.
Applications
Lab-on-a-chip
OSTE+ allows for soft lithography microstructuring, strong biocompatible dry bonding to almost any substrate during Lab-on-a-chip (LoC) manufacturing, while simultaneously mimicking the mechanical properties found in thermoplastic polymers, hence allowing for true prototyping of commercial LoC.[5] The commonly used materials for microfluidics suffer from unwieldy steps and often ineffective bonding processes, especially when packaging biofunctionalized surfaces, which makes LoC assembly difficult and costly [24][25] OSTE+ polymer which effectively bonds to nine dissimilar types of substrates, requires no surface treatment prior to the bonding at room temperature, features high Tg, and achieves good bonding strength to at least 100 °C.[5] Moreover, it has been demonstrated that excellent results can be obtained using photolithography on OSTE polymer, opening wider potential applications.[26]
Bio packaging
Biosensors are used for a range of different biological measurements, such as real time study of ligand- receptor kinetics or the rapid detection of biomarkers or pathogens.[27] The cartridge is made of a newly developed OSTE polymer which has an excess of thiol functional groups, allowing it to covalently bond to the gold surface of the sensor. The fabrication method of the OSTE cartridges is based on replica molding with rapid UV-curing and is therefore applicable to micro structured components and scalable up to high volume fabrication. The process addresses the need for a simple and straightforward production of robust, low cost and disposable sensor chips and eliminates many of the limitations of previous methods where PDMS molds were used.[28] The benefits of the novel method include that it allows for one step integration at low temperature in a dry environment, is uncomplicated and rapid, and eliminates the need for clamping, the necessity of manual assembly, the use of elastic and biocompatible cartridge materials, or surface activation/coating. OSTE packaging for biosensing has been demonstrated for QCM,[29] and photonic ring resonator sensors.[30]
Wafer bonding
Adhesive wafer bonding has become an established technology in microelectromechanical systems (MEMS) integration and packaging applications.[31] OSTE is suitable for heterogeneous silicon wafer level integration depending on its application in low temperature processes due to its ability to cure even in room temperatures.[32]
References
- ↑ MICROFLUIDICS & LAB-ON-CHIP
- ↑ Lab Chip , 2011 , 11, 3136
- ↑ Mercene Labs AB homepage
- ↑ Lab Chip , 2011 , 11, 3136
- 1 2 3 Saharil , Lab Chip 12, 3032-3035 (2012)
- ↑ Vastesson, Proc. IEEE Transducers 2013 Barcelona, 408-411 (2013)
- ↑ C.E.Hoyle and C.N.Bowman,Thiol-ene click chemistry, Angew. Chem. Int. Ed., 2010, 49, 1540
- 1 2 3 text of the citation
- ↑ Saharil, Journal of Micromechanics and Microengineering 23, 025021 (2013)
- ↑ BIOMICROFLUIDICS 6, 016505 (2012)
- ↑ Lafleur , Analyst 138, 845-849 (2013)
- ↑ OSTE+ Official datasheet
- ↑ Errando-Herranz, Proc. MicroTAS 2013 Freiburg, (2013)
- 1 2 Hansson, Proc. IEEE MEMS 2014 San Francisco, (2014)
- ↑ Zhou, Proc. MicroTAS 2013 Freiburg, (2013)
- ↑ C. E. Hoyle, T. Y. Lee and T. Roper, Thiol-enes: chemistry of the past with promise for the future,J. Polym. Sci., Part A: Polym. Chem.,2004, 42, 5301.,
- ↑ Saharil, Journal of Micromechanics and Microengineering 23, 025021 (2013)
- ↑ Sandström, N; Shafagh, R Z; Vastesson, A; Carlborg, C F; Wijngaart, W van der; Haraldsson, T. "Reaction injection molding and direct covalent bonding of OSTE+ polymer microfluidic devices". Journal of Micromechanics and Microengineering. 25 (7). doi:10.1088/0960-1317/25/7/075002.
- ↑ Sticker, Drago; Rothbauer, Mario; Lechner, Sarah; Hehenberger, Marie-Therese; Ertl, Peter (2015-11-24). "Multi-layered, membrane-integrated microfluidics based on replica molding of a thiol–ene epoxy thermoset for organ-on-a-chip applications". Lab Chip. 15 (24): 4542–4554. doi:10.1039/c5lc01028d. ISSN 1473-0189.
- ↑ Carlborg, Carl Fredrik; Haraldsson, Tommy; Öberg, Kim; Malkoch, Michael; Wijngaart, Wouter van der (2011-09-21). "Beyond PDMS: off-stoichiometry thiol–ene (OSTE) based soft lithography for rapid prototyping of microfluidic devices". Lab on a Chip. 11 (18). doi:10.1039/c1lc20388f. ISSN 1473-0189.
- ↑ Fredrik, Carlborg, Carl; M., Cretich,; Tommy, Haraldsson,; L., Sola,; M., Bagnati,; M., Chiari,; Wouter, van der Wijngaart, (2011-01-01). "Biosticker : patterned microfluidic stickers for rapid integration with microarrays".
- ↑ Hillmering, Mikael; Pardon, Gaspard; Vastesson, Alexander; Supekar, Omkar; Carlborg, Carl Fredrik; Brandner, Birgit D.; Wijngaart, Wouter van der; Haraldsson, Tommy (2016-02-15). "Off-stoichiometry improves the photostructuring of thiol–enes through diffusion-induced monomer depletion". Microsystems & Nanoengineering. 2. doi:10.1038/micronano.2015.43. ISSN 2055-7434.
- ↑ Hansson, Jonas; Yasuga, Hiroki; Haraldsson, Tommy; Wijngaart, Wouter van der (2016-01-05). "Synthetic microfluidic paper: high surface area and high porosity polymer micropillar arrays". Lab Chip. 16 (2): 298–304. doi:10.1039/c5lc01318f. ISSN 1473-0189.
- ↑ J. Micromech. Microeng. 18 (2008) 067001 (4pp)
- ↑ J. Micromech. Microeng. 21 (2011) 025008 (8pp)
- ↑ 1. Pardon G, et al., Microfluidics and Nanofluidics. 2014 Feb 14.
- ↑ Homola, Chemical Reviews, 108 (2), 462–493, 2008
- ↑ Carlborg, Proc. MicroTAS 2011 Seatle, 311-313 (2011)
- ↑ Sandström, Proc. IEEE Transducers 2011 Beijing, 2778-2781 (2011)
- ↑ Errando-Herranz, Opt. Express 21, 21293 (2013)
- ↑ Niklaus F, Stemme G, Lu J-Q and Gutmann R J 2006 Adhesive wafer bonding J. Appl. Phys. 99 03110
- ↑ Forsberg, Journal of Micromechanics and Microengineering 23, 085019 (2013)