PF-4840154
 | |
| Names | |
|---|---|
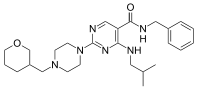
| IUPAC name
4-Isobutylamino-2-[4-(tetrahydro-pyran-3-ylmethyl)-piperazin-1-yl]-pyrimidine-5-carboxylic acid benzylamide | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL1818218 |
| ChemSpider | 26610754 |
| 6309 | |
| |
| |
| Properties | |
| C26H38N6O2 | |
| Molar mass | 466.63 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
PF-4840154 is pyrimidine derivative discovered by Pfizer at its Sandwich, Kent research center. The compound is a potent, selective activator of both the human (EC50 = 23 nM) and rat (EC50 = 97 nM) TRPA1 channels. This compound elicits nociception in a mouse model through TRPA1 activation. PF-4840154 is used as a reference agonist of the TRPA1 channel for in-vitro High-throughput screening purposes, and is superior to allyl isothiocyanate for this use.[1] The TRPA1 channel is considered an attractive pain target based on the fact that TRPA1 knockout mice showed near complete attenuation of pain behaviors in some pre-clinical development models.[2][3]
References
- ↑ Ryckmans T, Aubdool AA, Bodkin JV, Cox P, Brain SD, Dupont T, Fairman E, Hashizume Y, Ishii N, Kato T, Kitching L, Newman J, Omoto K, Rawson D, Strover J (July 2011). "Design and pharmacological evaluation of PF-4840154, a non-electrophilic reference agonist of the TrpA1 channel". Bioorganic. Med. Chem. Lett. (21): 4857–4859. doi:10.1016/j.bmcl.2011.06.035. PMID 21741838.
- ↑ McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM (August 2007). "TRPA1 mediates formalin-induced pain". Proc. Natl. Acad. Sci. U.S.A. 104 (33): 13525–30. doi:10.1073/pnas.0705924104. PMC 1941642
 . PMID 17686976.
. PMID 17686976. - ↑ McMahon SB, Wood JN (March 2006). "Increasingly irritable and close to tears: TRPA1 in inflammatory pain". Cell. 124 (6): 1123–5. doi:10.1016/j.cell.2006.03.006. PMID 16564004.
This article is issued from Wikipedia - version of the 6/4/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.