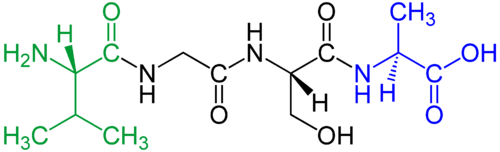
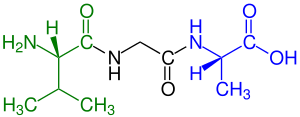Peptide
Peptides (from Gr.: πεπτός, peptós "digested"; derived from πέσσειν, péssein "to digest") are biologically occurring short chains of amino acid monomers linked by peptide (amide) bonds.
The covalent chemical bonds are formed when the carboxyl group of one amino acid reacts with the amine group of another. The shortest peptides are dipeptides, consisting of 2 amino acids joined by a single peptide bond, followed by tripeptides, tetrapeptides, etc. A polypeptide is a long, continuous, and unbranched peptide chain. Hence, peptides fall under the broad chemical classes of biological oligomers and polymers, alongside nucleic acids, oligosaccharides and polysaccharides, etc.
Peptides are distinguished from proteins on the basis of size, and as an arbitrary benchmark can be understood to contain approximately 50 or fewer amino acids.[1][2] Proteins consist of one or more polypeptides arranged in a biologically functional way, often bound to ligands such as coenzymes and cofactors, or to another protein or other macromolecule (DNA, RNA, etc.), or to complex macromolecular assemblies.[3] Finally, while aspects of the lab techniques applied to peptides versus polypeptides and proteins differ (e.g., the specifics of electrophoresis, chromatography, etc.), the size boundaries that distinguish peptides from polypeptides and proteins are not absolute: long peptides such as amyloid beta have been referred to as proteins, and smaller proteins like insulin have been considered peptides.
Amino acids that have been incorporated into peptides are termed "residues" due to the release of either a hydrogen ion from the amine end or a hydroxyl ion from the carboxyl end, or both, as a water molecule is released during formation of each amide bond.[4] All peptides except cyclic peptides have an N-terminal and C-terminal residue at the end of the peptide (as shown for the tetrapeptide in the image).
Peptide classes
Peptides are divided into several classes, depending on how they are produced:
- Milk peptides
- Two naturally occurring milk peptides are formed from the milk protein casein when digestive enzymes break this down; they can also arise from the proteinases formed by lactobacilli during the fermentation of milk.[5]
- Ribosomal peptides
- Ribosomal peptides are synthesized by translation of mRNA. They are often subjected to proteolysis to generate the mature form. These function, typically in higher organisms, as hormones and signaling molecules. Some organisms produce peptides as antibiotics, such as microcins.[6] Since they are translated, the amino acid residues involved are restricted to those utilized by the ribosome.
However, these peptides frequently have posttranslational modifications... such as phosphorylation, hydroxylation, sulfonation, palmitoylation, glycosylation and disulfide formation. In general, they are linear, although lariat structures have been observed.[7] More exotic manipulations do occur, such as racemization of L-amino acids to D-amino acids in platypus venom.[8]
- Nonribosomal peptides
- Nonribosomal peptides are assembled by enzymes that are specific to each peptide, rather than by the ribosome. The most common non-ribosomal peptide is glutathione, which is a component of the antioxidant defenses of most aerobic organisms.[9] Other nonribosomal peptides are most common in unicellular organisms, plants, and fungi and are synthesized by modular enzyme complexes called nonribosomal peptide synthetases.[10]
These complexes are often laid out in a similar fashion, and they can contain many different modules to perform a diverse set of chemical manipulations on the developing product.[11] These peptides are often cyclic and can have highly complex cyclic structures, although linear nonribosomal peptides are also common. Since the system is closely related to the machinery for building fatty acids and polyketides, hybrid compounds are often found. The presence of oxazoles or thiazoles often indicates that the compound was synthesized in this fashion.[12]
- Peptones
- See also Tryptone
- Peptones are derived from animal milk or meat digested by proteolysis.[13] In addition to containing small peptides, the resulting spray-dried material includes fats, metals, salts, vitamins and many other biological compounds. Peptones are used in nutrient media for growing bacteria and fungi.[14]
- Peptide fragments
- Peptide fragments refer to fragments of proteins that are used to identify or quantify the source protein.[15] Often these are the products of enzymatic degradation performed in the laboratory on a controlled sample, but can also be forensic or paleontological samples that have been degraded by natural effects.[16][17]
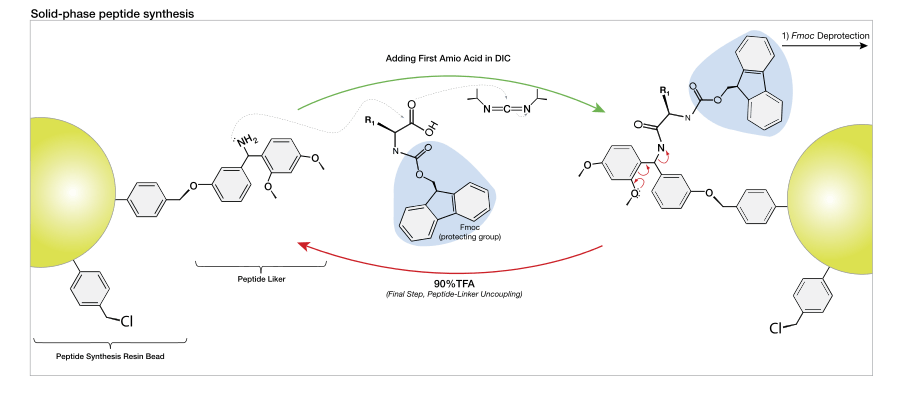
Peptide synthesis
Peptides in molecular biology
Peptides received prominence in molecular biology for several reasons. The first is that peptides allow the creation of peptide antibodies in animals without the need of purifying the protein of interest.[18] This involves synthesizing antigenic peptides of sections of the protein of interest. These will then be used to make antibodies in a rabbit or mouse against the protein.
Another reason is that peptides have become instrumental in mass spectrometry, allowing the identification of proteins of interest based on peptide masses and sequence.
Peptides have recently been used in the study of protein structure and function. For example, synthetic peptides can be used as probes to see where protein-peptide interactions occur- see the page on Protein tags.
Inhibitory peptides are also used in clinical research to examine the effects of peptides on the inhibition of cancer proteins and other diseases.[19] For example, one of the most promising application is through peptides that target LHRH.[20] These particular peptides act as an agonist, meaning that they bind to a cell in a way that regulates LHRH receptors. The process of inhibiting the cell receptors suggests that peptides could be beneficial in treating prostate cancer. However, additional investigations and experiments are required before the cancer-fighting attributes, exhibited by peptides, can be considered definitive.[21]
Well-known peptide families
The peptide families in this section are ribosomal peptides, usually with hormonal activity. All of these peptides are synthesized by cells as longer "propeptides" or "proproteins" and truncated prior to exiting the cell. They are released into the bloodstream where they perform their signaling functions.
Antimicrobial peptides
- Magainin family
- Cecropin family
- Cathelicidin family
- Defensin family
Tachykinin peptides
Vasoactive intestinal peptides
- VIP (Vasoactive Intestinal Peptide; PHM27)
- PACAP Pituitary Adenylate Cyclase Activating Peptide
- Peptide PHI 27 (Peptide Histidine Isoleucine 27)
- GHRH 1-24 (Growth Hormone Releasing Hormone 1-24)
- Glucagon
- Secretin
Pancreatic polypeptide-related peptides
Opioid peptides
- Proopiomelanocortin (POMC) peptides
- Enkephalin pentapeptides
- Prodynorphin peptides
Calcitonin peptides
Other peptides
- B-type Natriuretic Peptide (BNP) - produced in myocardium & useful in medical diagnosis
- Lactotripeptides - Lactotripeptides might reduce blood pressure,[22][23][24] although the evidence is mixed.[25]
Notes on terminology
Length:
- A polypeptide is a single linear chain of many amino acids, held together by amide bonds.
- A protein is one or more polypeptide (more than about 50 amino acids long).
- An oligopeptide consists of only a few amino acids (between two and twenty).
Number of amino acids:
- A monopeptide has one amino acid.
- A dipeptide has two amino acids.
- A tripeptide has three amino acids.
- A tetrapeptide has four amino acids.
- A pentapeptide has five amino acids.
- A hexapeptide has six amino acids.
- A heptapeptide has seven amino acids.
- An octapeptide has eight amino acids (e.g., angiotensin II).
- A nonapeptide has nine amino acids (e.g., oxytocin).
- A decapeptide has ten amino acids (e.g., gonadotropin-releasing hormone & angiotensin I).
- An undecapeptide (or monodecapeptide) has eleven amino acids, a dodecapeptide (or didecapeptide) has twelve amino acids, a tridecapeptide has thirteen amino acids, and so forth.
- An icosapeptide has twenty amino acids, a tricontapeptide has thirty amino acids, a tetracontapeptide has forty amino acids, and so forth.
Function:
- A neuropeptide is a peptide that is active in association with neural tissue.
- A lipopeptide is a peptide that has a lipid connected to it, and pepducins are lipopeptides that interact with GPCRs.
- A peptide hormone is a peptide that acts as a hormone.
- A proteose is a mixture of peptides produced by the hydrolysis of proteins. The term is somewhat archaic.
Doping in sports
The term peptide has been used to mean secretagogue peptides and peptide hormones in sports doping matters: secretagogue peptides are classified as Schedule 2 (S2) prohibited substances on the World Anti-Doping Agency (WADA) Prohibited List, and are therefore prohibited for use by professional athletes both in and out of competition. Such secretagogue peptides have been on the WADA prohibited substances list since at least 2008. The Australian Crime Commission cited the alleged misuse of secretagogue peptides in Australian sport including growth hormone releasing peptides CJC-1295, GHRP-6, and GHSR (gene) hexarelin. There is ongoing controversy on the legality of using secretagogue peptides in sports.[26]
See also
| Wikiquote has quotations related to: Peptide |
- Argireline
- Beefy meaty peptide
- Bis-peptide
- Epidermal Growth Factor
- Journal of Peptide Science
- Lactotripeptides
- Multifunctional peptides
- Neuropeptides
- Palmitoyl pentapeptide-4
- Pancreatic hormone
- Peptide Spectral Library
- Peptide synthesis
- Peptidomimetics (such as peptoids and β-peptides) to peptides, but with different properties.
- Protein tag, describing addition of peptide sequences to enable protein isolation or detection
- Ribosome
- Translation
References
- ↑ IUPAC. Compendium of Chemical Terminology, 2nd ed. (the "Gold Book"). Compiled by A. D. McNaught and A. Wilkinson. Blackwell Scientific Publications, Oxford (1997). XML on-line corrected version: http://goldbook.iupac.org (2006-) created by M. Nic, J. Jirat, B. Kosata; updates compiled by A. Jenkins. ISBN 0-9678550-9-8. doi:10.1351/goldbook.P04898.
- ↑ "What are peptides". Zealand Pharma A/S. Archived from the original on 2014-07-14.
- ↑ Ardejani, Maziar S.; Orner, Brendan P. (2013-05-03). "Obey the Peptide Assembly Rules". Science. 340 (6132): 561–562. doi:10.1126/science.1237708. ISSN 0036-8075. PMID 23641105.
- ↑ IUPAC, Compendium of Chemical Terminology, 2nd ed. (the "Gold Book") (1997). Online corrected version: (2006–) "amino-acid residue in a polypeptide".
- ↑ Haque, Emily; Chand, Rattan. "Milk protein derived bioactive peptides". Dairy Science. Retrieved 28 July 2014.
- ↑ Duquesne S, Destoumieux-Garzón D, Peduzzi J, Rebuffat S; Destoumieux-Garzón; Peduzzi; Rebuffat (August 2007). "Microcins, gene-encoded antibacterial peptides from enterobacteria". Natural Product Reports. 24 (4): 708–34. doi:10.1039/b516237h. PMID 17653356.
- ↑ Pons M, Feliz M, Antònia Molins M, Giralt E; Feliz; Antònia Molins; Giralt (May 1991). "Conformational analysis of bacitracin A, a naturally occurring lariat". Biopolymers. 31 (6): 605–12. doi:10.1002/bip.360310604. PMID 1932561.
- ↑ Torres AM, Menz I, Alewood PF, et al. (July 2002). "D-Amino acid residue in the C-type natriuretic peptide from the venom of the mammal, Ornithorhynchus anatinus, the Australian platypus". FEBS Letters. 524 (1–3): 172–6. doi:10.1016/S0014-5793(02)03050-8. PMID 12135762.
- ↑ Meister A, Anderson ME; Anderson (1983). "Glutathione". Annual Review of Biochemistry. 52 (1): 711–60. doi:10.1146/annurev.bi.52.070183.003431. PMID 6137189.
- ↑ Hahn M, Stachelhaus T; Stachelhaus (November 2004). "Selective interaction between nonribosomal peptide synthetases is facilitated by short communication-mediating domains". Proceedings of the National Academy of Sciences of the United States of America. 101 (44): 15585–90. Bibcode:2004PNAS..10115585H. doi:10.1073/pnas.0404932101. PMC 524835
 . PMID 15498872.
. PMID 15498872. - ↑ Finking R, Marahiel MA; Marahiel (2004). "Biosynthesis of nonribosomal peptides1". Annual Review of Microbiology. 58 (1): 453–88. doi:10.1146/annurev.micro.58.030603.123615. PMID 15487945.
- ↑ Du L, Shen B; Shen (March 2001). "Biosynthesis of hybrid peptide-polyketide natural products". Current Opinion in Drug Discovery & Development. 4 (2): 215–28. PMID 11378961.
- ↑ http://www.usvpeptides.com
- ↑ Payne JW (1976). "Peptides and micro-organisms". Advances in Microbial Physiology. Advances in Microbial Physiology. 13: 55–113. doi:10.1016/S0065-2911(08)60038-7. ISBN 9780120277131. PMID 775944.
- ↑ Hummel J, Niemann M, Wienkoop S; et al. (2007). "ProMEX: a mass spectral reference database for proteins and protein phosphorylation sites". BMC Bioinformatics. 8 (1): 216. doi:10.1186/1471-2105-8-216. PMC 1920535
 . PMID 17587460.
. PMID 17587460. - ↑ Webster J, Oxley D; Oxley (2005). "Peptide mass fingerprinting: protein identification using MALDI-TOF mass spectrometry". Methods in Molecular Biology. Methods in Molecular Biology™. 310: 227–40. doi:10.1007/978-1-59259-948-6_16. ISBN 978-1-58829-399-2. PMID 16350956.
- ↑ Marquet P, Lachâtre G; Lachâtre (October 1999). "Liquid chromatography-mass spectrometry: potential in forensic and clinical toxicology". Journal of Chromatography B. 733 (1–2): 93–118. doi:10.1016/S0378-4347(99)00147-4. PMID 10572976.
- ↑ Bulinski JC (1986). "Peptide antibodies: new tools for cell biology". International Review of Cytology. International Review of Cytology. 103: 281–302. doi:10.1016/S0074-7696(08)60838-4. ISBN 9780123645036. PMID 2427468.
- ↑ Herce, Henry D.; Deng, Wen; Helma, Jonas; Leonhardt, Heinrich; Cardoso, M. Cristina (24 October 2013). "Visualization and targeted disruption of protein interactions in living cells". Nature Communications. 4. doi:10.1038/ncomms3660. PMC 3826628
 . PMID 24154492.
. PMID 24154492. - ↑ "Hormone (androgen deprivation) therapy for prostate cancer". cancer.org. Retrieved 3 October 2013.
- ↑ Kumar, Ravi; Barqawi, A; Crawford, ED (2005). "Adverse Events Associated with Hormonal Therapy for Prostate Cancer". Reviews in Urology. 7 (5): 37–43. PMC 1477613
 . PMID 16985883.
. PMID 16985883. - ↑ Boelsma E, Kloek J; Kloek (March 2009). "Lactotripeptides and antihypertensive effects: a critical review". The British Journal of Nutrition. 101 (6): 776–86. doi:10.1017/S0007114508137722. PMID 19061526.
- ↑ Xu JY, Qin LQ, Wang PY, Li W, Chang C; Qin; Wang; Li; Chang (October 2008). "Effect of milk tripeptides on blood pressure: a meta-analysis of randomized controlled trials". Nutrition. 24 (10): 933–40. doi:10.1016/j.nut.2008.04.004. PMID 18562172.
- ↑ Pripp AH (2008). "Effect of peptides derived from food proteins on blood pressure: a meta-analysis of randomized controlled trials". Food & Nutrition Research. 52: 10.3402/fnr.v52i0.1641. doi:10.3402/fnr.v52i0.1641. PMC 2596738
 . PMID 19109662.
. PMID 19109662. - ↑ Engberink MF, Schouten EG, Kok FJ, van Mierlo LA, Brouwer IA, Geleijnse JM; Schouten; Kok; Van Mierlo; Brouwer; Geleijnse (February 2008). "Lactotripeptides show no effect on human blood pressure: results from a double-blind randomized controlled trial". Hypertension. 51 (2): 399–405. doi:10.1161/HYPERTENSIONAHA.107.098988. PMID 18086944.
- ↑ https://theconversation.edu.au/we-need-an-advocate-against-asadas-power-in-doping-control-12119