Polyvinyl toluene
Polyvinyltoluene (PVT, polyvinyl toluene) is a synthetic polymer of alkylbenzenes with a linear formula [CH2CH(C6H4CH3)]n. Commercial vinyl toluene is a mixture of methyl styrene isomers[1]
-
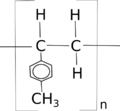
chemical formula for PVT
Uses
PVT can be doped with anthracene or other wavelength-shifting dopants [2] to produce a plastic scintillator. When subjected to ionizing radiation (both particle radiation and gamma radiation), the amount of visible radiation emitted is proportional to the absorbed dose as long as the energy loss per length is not too large. A relation applicable to a wide range of values for energy loss per unit length is given by Birks' Law.
PVT can be damaged by radiation with high stopping power, e.g. ion beams or by any kind of ionizing radiation. A review of radiation damage for PVT and other similar plastic scintillators can be found at.[3] Such radiation breaks the C-H bonds and creates color centers which absorb the produced light, significantly reduces the light output.[4]
References
- ↑ Birks, J.B. (1964). The Theory and Practice of Scintillation Counting. London: Pergamon.
- ↑ Birks, J.B. (1964). The Theory and Practice of Scintillation Counting. London: Pergamon.
- ↑ Sauli, F. (1993). Instrumentation in High Energy Physics (2nd ed.). World Scientific. ISBN 978-981-02-0597-3.
- ↑ Bross, A.; et al. (1992). "Radiation damage of plastic scintillators". IEEE Trans. Nucl. Sci. 39: 1199. doi:10.1109/23.173178.