Porphyria cutanea tarda
| Porphyria cutanea tarda | |
|---|---|
| Classification and external resources | |
| Specialty | endocrinology |
| ICD-10 | E80.1 |
| ICD-9-CM | 277.1 |
| OMIM | 176100 176100 |
| DiseasesDB | 10376 29123 |
| eMedicine | derm/344 |
| MeSH | D017119 |
Porphyria cutanea tarda (PCT) is the most common subtype of porphyria.[1] The disease is named because it is a porphyria that often presents with skin manifestations later in life. The disorder results from low levels of the enzyme responsible for the fifth step in heme production. Heme is a vital molecule for all of the body's organs. It is a component of hemoglobin, the molecule that carries oxygen in the blood.
Hepatoerythropoietic porphyria has been described as a homozygous form of porphyria cutanea tarda,[2] although it can also be caused if two different mutations occur at the same locus.
Signs and symptoms
Porphyria cutanea tarda (commonly referred to as PCT) is recognized as the most prevalent subtype of porphyritic diseases.[3]
The disease is characterized by onycholysis and blistering of the skin in areas that receive higher levels of exposure to sunlight. The primary cause of this disorder is a deficiency of uroporphyrinogen decarboxylase (UROD), a cytosolic enzyme that is a step in the enzymatic pathway that leads to the synthesis of heme. While a deficiency in this enzyme is the direct cause leading to this disorder, there are a number of both genetic and environmental risk factors that are associated with PCT.[4]
Typically, patients who are ultimately diagnosed with PCT first seek treatment following the development of photosensitivities in the form of blisters and erosions on commonly exposed areas of the skin. This is usually observed in the face, hands, forearms, and lower legs. It heals slowly and with scarring. Though blisters are the most common skin manifestations of PCT, other skin manifestations like hyperpigmentation (as if they are getting a tan) and hypertrichosis (mainly on top of the cheeks) also occur. PCT is a chronic condition, with external symptoms often subsiding and recurring as a result of a number of factors. In addition to the symptomatic manifestation of the disease in the skin, chronic liver problems are extremely common in patients with the sporadic form of PCT. These include hepatic fibrosis (scarring of the liver), cirrhosis, and inflammation. However, liver problems are less common in patients with the inherited form of the disease.[5] Additionally, patients will often void a wine-red color urine with an increased concentration of uroporphyrin I due to their enzymatic deficiency.[6]
Cause
Genetics
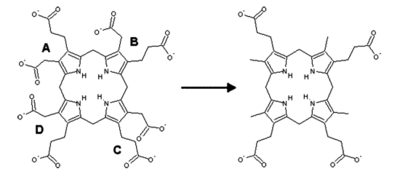
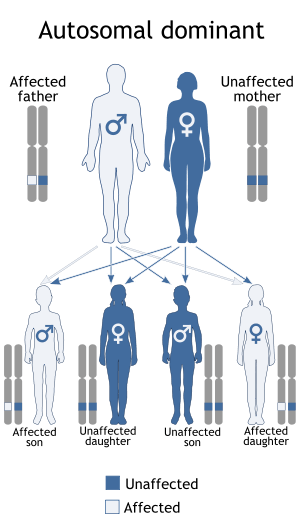
Inherited mutations in the UROD gene cause about 20% of cases (the other 80% of cases do not have mutations in UROD, and are classified as sporadic). UROD makes an enzyme called uroporphyrinogen III decarboxylase, which is critical to the chemical process that leads to heme production. The activity of this enzyme is usually reduced by 50% in all tissues in people with the inherited form of the condition.
Nongenetic factors such as alcohol abuse, excess iron, and others listed above can increase the demand for heme and the enzymes required to make heme. The combination of this increased demand and reduced activity of uroporphyrinogen decarboxylase disrupts heme production and allows byproducts of the process to accumulate in the body, triggering the signs and symptoms of porphyria cutanea tarda.
The HFE gene makes a protein that helps cells regulate the absorption of iron from the digestive tract and into the cells of the body. Certain mutations in the HFE gene cause hemochromatosis (an iron overload disorder). People who have these mutations are also at an increased risk of developing porphyria cutanea tarda.
In the 20% of cases where porphyria cutanea tarda is inherited, it is inherited in an autosomal dominant pattern, which means one copy of the altered gene is sufficient to decrease enzyme activity and cause the signs and symptoms of the disorder.
Other
While inherited deficiencies in uroporphyrinogen decarboxylase often lead to the development of PCT, there are a number of risk factors that can both cause and exacerbate the symptoms of this disease. One of the most common risk factors observed is infection with the Hepatitis C virus.[7] One review of a collection of PCT studies noted Hepatitis C infection in 50% of documented cases of PCT. Additional risk factors include alcohol abuse, excess iron, and exposure to chlorinated cyclic hydrocarbons and Agent Orange.
It can be a paraneoplastic phenomenon.[8]
Exacerbating factors
Pathogenesis
Porphyria cutanea tarda is primarily caused by uroporphyrinogen decarboxylase deficiency (UROD). Uroporphyrinogen decarboxylase occurs in nature as a homodimer of two subunits. It participates in the fifth step in heme synthesis pathway, and is active in the cytosol. This enzymatic conversion results in coproporphyrinogen III as the primary product. This is accomplished by the clockwise removal of the four carboxyl groups present in the cyclic uroporphyrinogen III molecule. Therefore, a deficiency in this enzyme causes the aforementioned buildup of uroporphyrinogen in the urine, which can be helpful in the diagnosis of this disorder.
The dermatological symptoms of PCT that include blistering and lesions on sun-exposed areas of the skin are caused by a buildup of porphyrin compounds (specifically uroporphyrinogen) close to the surface of the skin. Due to the highly conjugated structure of porphyrins involving alternating single and double carbon bonds, these compounds exhibit a deep purple color, resulting in the discoloration observed in the skin. The excess uroporphyrinogen that leads to these lesions is primarily produced in the liver, but exposure to excess sunlight is thought to lead to the production of reactive oxygen species, disrupting the activity of uroporphyrinogen decarboxylase and contributing to the buildup of uroporphyrinogen. This is thought to be the underlying biochemical pathway behind the photosensitivity observed in patients with PCT. The strong association of PCT with Hepatitis C infection is not entirely understood. Studies have suggested that the cytopathic effect of the virus on hepatocytes can lead to the release of free iron. This iron can disrupt the activity of cytochrome p450, releasing activated oxygen species. These can oxidize the UROD substrate uroporphyrinogen, which can result in the inhibition of UROD and lead to deficient activity of this key enzyme.
Excess alcohol abuse is frequently associated with both inducing PCT[11] and aggravating a preexisting diagnosis of the disorder. It is thought to do so by causing oxidative damage to liver cells, resulting in oxidized species of uroporphyrinogen that inhibit the activity of hepatic UROD. It is also felt to increase the uptake of iron in liver cells, leading to further oxidation of uroporphyrinogen by the release of activated oxygen species. Additionally, exposure to chlorinated cyclic hydrocarbons can lead to a deficiency in the activity of uroporphyrinogen decarboxylase, causing the buildup of excess uroporphyrinogen. Additionally, alcohol has been shown to increase the activity of the delta-aminolevulinic acid synthetase (ALA synthetase), the rate-limiting enzymatic step in heme synthesis in the mitochondria, in rats.[12] Therefore, alcohol consumption may increase the production of uroporphyrinogen, exacerbating symptoms in individuals with porphyria cutanea tarda.
Diagnosis
While the most common symptom of PCT is the appearance of skin lesions and blistering, their appearance does not single-handedly lead to a conclusive diagnosis. Laboratory testing will commonly reveal high levels of uroporphyrinogen in the urine, clinically referred to as uroporphyrinogenuria. Additionally, testing for common risk factors such as Hepatitis C and hemochromatosis is strongly suggested, as their high prevalence in patients with PCT may require additional treatment. If clinical appearance of PCT is present, but laboratories are negative, one needs to seriously consider the diagnosis of pseudoporphyria.
Classification
Some sources divide PCT into two types: sporadic and familial.[2] Other sources include a third type,[13] but this is less common.
| Type | OMIM | Description |
|---|---|---|
| Type I ("sporadic") | 176090 | Type I porphyria cutanea tarda, the sporadic form, is indicated by UROD deficiency that is observed only in hepatic cells and nowhere else in the body. Genetically, these individuals will not exhibit deficiency in the UROD gene, although other genetic factors such as HFE deficiency (resulting in hemochromatosis and the buildup of iron in the liver) are thought to play a key role. Typically in these individuals, a variety of risk factors such as alcohol abuse and Hepatitis C infection conspire to result in the clinical manifestation of PCT. |
| Type II ("familial") | 176100 | Patients exhibiting Type II PCT have a specific deficiency in the UROD gene, passed down in an autosomal dominant pattern. Those possessing this deficiency are heterozygous for the UROD gene. They do not show a complete lack of functional uroporphyrinogen decarboxylase, only a deficient form of the enzyme that is marked by reduced conversion of uroporphyrinogen to coproporphyrinogen. Therefore, the expression of uroporphyrinogen decarboxylase will be reduced throughout the body of these individuals, while it is isolated to the liver in Type I patients. While this genetic deficiency is the main distinction between Type I and Type II PCT, the risk factors mentioned before are often seen in patients presenting with Type II PCT. In fact, many people who possess the deficient UROD gene often go their entire lives without having a clinical manifestation of PCT symptoms. |
| Type III | - | The least common is Type III, which is no different from Type I insofar as the patients possess normal UROD genes. Despite this, Type III PCT is observed in more than one family member, indicating a genetic component unrelated to the expression of uroporphyrinogen decarboxylase. |
One study used 74% as the cutoff for UROD activity, with those patients under that number being classified as type II, and those above classified as type III if there was a family history, and type I if there was not.[14]
Genetic variants associated with hemochromatosis have been observed in PCT patients,[9] which may help explain inherited PCT not associated with UROD.
Treatment
Since PCT is a chronic condition, a comprehensive management of the disease is the most effective means of treatment. Primarily, it is key that patients diagnosed with PCT avoid alcohol consumption, iron supplements, excess exposure to sunlight (especially in the summer), as well as estrogen and chlorinated cyclic hydrocarbons, all of which can potentially exacerbate the disorder. Additionally, the management of excess iron (due to the commonality of hemochromatosis in PCT patients) can be achieved through phlebotomy, whereby blood is systematically drained from the patient. Low doses of antimalarials can be used. They remove excess porphyrins from the liver by increasing the excretion rate. Remission can be seen within 6–12 months. Originally, higher doses were used to treat the condition but are no longer recommended because of liver toxicity.[15] Finally, due to the strong association between PCT and Hepatitis C, the treatment of Hepatitis C (if present) is vital to the effective treatment of PCT. Chloroquine, hydroxychloroquine, and venesection are typically employed in the management strategy.[16]
Epidemiology
Porphyria cutanea tarda has a prevalence estimated at approximately 1 in 10,000.[17] An estimated 80% of porphyria cutanea tarda cases are sporadic. The exact frequency is not clear because many people with the condition never experience symptoms.
Society and culture
Porphyria cutanea tarda is implicated in the origin of vampire myths. This is because people with the disease tend to avoid the sun due to photosensitivity (and therefore have a young complexion and pallor) and require blood transfusions. Hypertrichosis (i.e. A full head of hair) will also give a young appearance.
Porphyria Cutanea Tarda is the name of a song by the rock band AFI on their fourth album Black Sails in the Sunset, released on May 18, 1999.
References
- ↑ Phillips JD, Bergonia HA, Reilly CA, Franklin MR, Kushner JP (March 2007). "A porphomethene inhibitor of uroporphyrinogen decarboxylase causes porphyria cutanea tarda". Proc. Natl. Acad. Sci. U.S.A. 104 (12): 5079–84. doi:10.1073/pnas.0700547104. PMC 1820519
 . PMID 17360334.
. PMID 17360334. - 1 2 "porphyria cutanea tarda" at Dorland's Medical Dictionary
- ↑ Danton M, Lim CK (July 2007). "Porphomethene inhibitor of uroporphyrinogen decarboxylase: analysis by high-performance liquid chromatography/electrospray ionization tandem mass spectrometry". Biomed. Chromatogr. 21 (7): 661–3. doi:10.1002/bmc.860. PMID 17516469.
- ↑ Kushner, J; et al. (1976). "An inherited enzymatic defect in porphyria cutanea tarda: decreased uroporphyrinogen decarboxylase activity". J Clin Invest. 58 (5): 1089–97. doi:10.1172/JCI108560. PMC 333275
 . PMID 993332.
. PMID 993332. - ↑ DiPadova, C; et al. (1983). "Effects of phlebotomy on urinary porphyrin pattern and liver histology in patients with porphyria cutanea tarda". Am J Med Sci. 285 (1): 2–12. doi:10.1097/00000441-198301000-00001. PMID 6824014.
- ↑ Goljan, E. F. (2011). Pathology (3rd ed., rev. reprint.). Philadelphia, PA: Mosby/Elsevier.
- ↑ Azim J, McCurdy H, Moseley RH (October 2008). "Porphyria cutanea tarda as a complication of therapy for chronic hepatitis C". World J. Gastroenterol. 14 (38): 5913–5. doi:10.3748/wjg.14.5913. PMC 2751904
 . PMID 18855993. Archived from the original on 2009-02-27.
. PMID 18855993. Archived from the original on 2009-02-27. - ↑ Sökmen M, Demırsoy H, Ersoy O, et al. (September 2007). "Paraneoplastic porphyria cutanea tarda associated with cholangiocarcinoma: Case report". Turk J Gastroenterol. 18 (3): 200–205. PMID 17891697. Archived from the original on 2011-07-28.
- 1 2 Frank J, Poblete-Gutiérrez P, Weiskirchen R, Gressner O, Merk HF, Lammert F (2006). "Hemochromatosis gene sequence deviations in German patients with porphyria cutanea tarda" (PDF). Physiol Res. 55 (Suppl 2): S75–83. PMID 17298224.
- ↑ Sampietro M, Fiorelli G, Fargion S (March 1999). "Iron overload in porphyria cutanea tarda". Haematologica. 84 (3): 248–53. PMID 10189391.
- ↑ "eMedicine/Stedman Medical Dictionary Lookup!". Retrieved 2008-12-04.
- ↑ Held H (1977). "Effect of alcohol on the heme and porphyrin synthesis interaction with phenobarbital and pyrazole". Digestion. 15: 136–46. doi:10.1159/000197995. PMID 838185.
- ↑ Méndez M, Poblete-Gutiérrez P, García-Bravo M, et al. (September 2007). "Molecular heterogeneity of familial porphyria cutanea tarda in Spain: characterization of 10 novel mutations in the UROD gene". Br. J. Dermatol. 157 (3): 501–7. doi:10.1111/j.1365-2133.2007.08064.x. PMID 17627795.
- ↑ Cruz-Rojo J, Fontanellas A, Morán-Jiménez MJ, et al. (December 2002). "Precipitating/aggravating factors of porphyria cutanea tarda in Spanish patients". Cell. Mol. Biol. (Noisy-le-grand). 48 (8): 845–52. PMID 12699242.
- ↑ http://www.clinuvel.com/skin-conditions/rare-skin-conditions/porphyria-cutanea-tarda-pct
- ↑ Sarkany RP (2001). "The management of porphyria cutanea tarda". Clin Exp Dermatol. 26 (3): 225–32. doi:10.1046/j.1365-2230.2001.00825.x. PMID 11422163.
- ↑ Arceci, Robert.; Hann, Ian M.; Smith, Owen P. (2006). Pediatric hematolog. Malden MA: Blackwell. ISBN 978-1-4051-3400-2.
External links
- Porphyria cutanea tarda at NIH's Office of Rare Diseases
- www.porphyria-europe.com