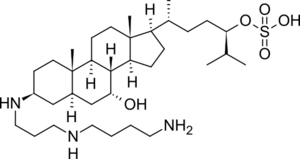
Squalamine
 | |
| Names | |
|---|---|
| IUPAC name
(1S,2S,5S,7R,9R,10R,11S,14R,15R)-N-{3-[(4- aminobutyl)amino]propyl}-9-hydroxy-2,15-dimethyl- 14-[(2R,5R)-6-methyl-5-(sulfooxy)heptan-2- yl]tetracyclo[8.7.0.0^{2,7}.0^{11,15}]heptadecan-5- aminium | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| ChEMBL | ChEMBL444929 ChEMBL507931 |
| ChemSpider | 65407 |
| PubChem | 72495 |
| UNII | F8PO54Z4V7 |
| |
| |
| Properties | |
| C34H65N3O5S | |
| Molar mass | 628 g/mol |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Squalamine /ˈskweɪləmiːn/[1] is an aminosterol compound with potent broad spectrum antimicrobial activity discovered in the tissues of the dogfish shark (Squalus acanthias) by a team led by Michael Zasloff.[2] Dr. Zasloff searched the tissues of the dogfish for compounds that might help explain the hardiness of this animal to infection, despite its "primitive" immune system. Using techniques that Zasloff's team had developed to isolate and identify antimicrobial peptides from animal tissues, the team extracted and characterized a novel bile acid-like compound containing a polyamine never before seen in nature.[3][4] The compound was called "squalamine", based on its source (Squalus acanthias) and its chemical structure (a sterol linked to a polyamine). Further analyses of larger quantities of dogfish liver extracts revealed squalamine to be the most abundant member of a larger aminosterol family comprising at least 12 related compounds.[5] One of these, "MSI-1436" or trodusquemine, although structurally similar to squalamine (it carries a spermine rather than a spermidine) and also quite potent as an anti-infective, exhibits a profoundly different pharmacology in vertebrates, causing weight loss and adipose tissue mobilization.[6] Squalamine is sometimes confused with the similar-sounding squalene, an unrelated compound also found in shark liver.
Pharmacology
Squalamine was initially discovered on the basis of its anti-bacterial activity. It has proven to be a broad spectrum antimicrobial compound that exhibits potent activity in vitro and in vivo against gram negative and gram positive bacteria,[7] fungi,[8] protozoa, and many viruses.[9] Subsequent studies in vitro and in animals demonstrated various unanticipated pharmacological properties. In several vertebrate models, squalamine exhibits systemic anti-angiogenic activity against rapidly proliferating blood vessels that arise in pathological settings. As a consequence it is being evaluated in several human clinical trials for cancer, macular degeneration, diabetic retinopathy, and fibrodysplasia ossificans progressiva. Ohr Pharmaceuticals is currently evaluating squalamine in a Phase II study for angiopathic retinopathy applied topically to the eye. In mammals, systemically administered squalamine is cleared by the liver, and transported via the biliary system into the feces. Squalamine has been evaluated in trials for treatment of non-small cell lung cancer (stage I/IIA), ovarian cancer (stage IV), and prostate cancer as well as several phase I pharmacokinetic studies.[10][11] In 2005, the Food and Drug Administration granted squalamine Fast Track status for approval for treatment of age-related macular degeneration.[12]
Sources
The most abundant natural source of squalamine is in the livers of dogfish and other species of the Squaliform sharks, though it is found in other sources, such as lampreys.[13] Squalamine was synthesized by a team from Magainin Pharmaceuticals headed by Kinney in 1995, and the synthetic compound has been used since that time for laboratory and clinical studies.
Mechanism of Action
Squalamine is an amphipathic zwitterionic molecule. At physiological pH it is positively charged. Because of the stereochemistry of its steroid framework, it assumes a flat, plate-like structure. As a consequence of these physical properties, squalamine binds electrostatically to membranes made of negatively charged phospholipids(PLs). The orientation of the polar substituents on squalamine causes it to sit on the surface of the membrane to which it binds, rather than penetrate the surface. Bacteria, unlike most eukaryotic cells, display negatively charged PLs on the membrane surfaces that are exposed to the environment. Squalamine, like many of the cationic antimicrobial peptides and proteins that have been discovered to date,[14] interacts with the plasma membranes of bacteria and disturb function; precisely how squalamine's membrane attack kills its microbial targets remains unknown. Eukaryotic cells position negatively charged PLs on the inner leaflet (the cytoplasmic face)of the plasma membrane;the outer leaflet, the surface exposed to the outside world, is populated principally by zwitterionic PLs, such as phosphatidyl choline. Why such asymmetry in the anionic phospholipid composition of the inner and outer leaflets of the plasma membrane exists is not known. Squalamine does not normally interact with the plasma membranes of normal eukaryotic cells because of the absence of negatively charged PLs on their surface. However, certain cells possess specific "transporters" that provide squalamine with an access through the membrane. Liver, capillary, and certain hematopoetic cells possess these transporters and define the pharmacology of the compound, restricting its bio-distribution to a limited numbers of tissues and organs in an animal. Once squalamine has traversed the plasma membrane, it binds electrostatically to the negatively charged cytoplasmic surface of the plasma membrane, effectively neutralizing the negative surface potential. As a consequence, proteins that are bound to that surface through electrostatic interactions are displaced. The cellular consequences of this "displacement" will vary depending upon the specific cellular context. Since proteins such as the Rho GTPases, which play a role in actin dynamics and cellular movement, are linked to the cytoplasmic surface of the plasma membrane, squalamine disrupts several Rac1-actin-dependent processes, such as growth factor dependent endothelial migration and angiogenesis.[9]
References
- ↑ US dict: skwā-lə-mēn
- ↑ Moore, K S; S Wehrli; H Roder; M Rogers; J N Forrest; D McCrimmon; M Zasloff (1993-02-15). "Squalamine: an aminosterol antibiotic from the shark". Proceedings of the National Academy of Sciences of the United States of America. 90 (4): 1354–8. doi:10.1073/pnas.90.4.1354. PMC 45871
 . PMID 8433993. Retrieved 2009-03-30.
. PMID 8433993. Retrieved 2009-03-30. - ↑ Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1354-8.
- ↑ Steroids. 1993 Aug;58(8):370-8.
- ↑ J Nat Prod. 2000 May;63(5):631-5.
- ↑ Int J Obes Relat Metab Disord. 2001 May;25(5):689-97.
- ↑ Curr Med Chem. 2010;17(32):3909-17
- ↑ Alhanout, K.; Brunel, J. M.; Ranque, S.; Rolain, J. M. (2010). "In vitro antifungal activity of aminosterols against moulds isolated from cystic fibrosis patients". Journal of Antimicrobial Chemotherapy. 65 (6): 1307–1309. doi:10.1093/jac/dkq089. PMID 20335187.
- 1 2 Zasloff, M.; Adams, A. P.; Beckerman, B.; Campbell, A.; Han, Z.; Luijten, E.; Meza, I.; Julander, J.; Mishra, A.; Qu, W.; Taylor, J. M.; Weaver, S. C.; Wong, G. C. L. (2011). "Squalamine as a broad-spectrum systemic antiviral agent with therapeutic potential". Proceedings of the National Academy of Sciences. 108 (38): 15978–15983. doi:10.1073/pnas.1108558108. PMC 3179074
 . PMID 21930925.
. PMID 21930925. - ↑ Herbst, R. S.; L. A. Hammond; D. P. Carbone; H. T. Tran; K. J. Holroyd; A. Desai; J. I. Williams; B. N. Bekele; H. Hait; V. Allgood (2003). "A Phase I/IIA Trial of Continuous Five-Day Infusion of Squalamine Lactate (MSI-1256F) Plus Carboplatin and Paclitaxel in Patients with Advanced Non-Small Cell Lung Cancer 1". Clinical Cancer Research. 9 (11): 4108–15. PMID 14519633.
- ↑ Hao, Desiree; Lisa A. Hammond, S. Gail Eckhardt, Amita Patnaik, Chris H. Takimoto, Garry H. Schwartz, Andrew D. Goetz, Anthony W. Tolcher, Heather A. McCreery, Khalid Mamun, Jon I. Williams, Kenneth J. Holroyd, Eric K. Rowinsky (2003-07-01). "A Phase I and Pharmacokinetic Study of Squalamine, an Aminosterol Angiogenesis Inhibitor". Clin Cancer Res. 9 (7): 2465–71. PMID 12855619. Retrieved 2009-03-30. Cite uses deprecated parameter
|coauthors=(help) - ↑ "CATE: California Assistive Technology Exchange". California Assistive Technology Exchange. Archived from the original on April 30, 2009. Retrieved 2009-03-31.
- ↑ Yun, Sang-Seon; Weiming Li (2007-12-01). "Identification of squalamine in the plasma membrane of white blood cells in the sea lamprey, Petromyzon marinus". J. Lipid Res. 48 (12): 2579–2586. doi:10.1194/jlr.M700294-JLR200. PMID 17726196. Retrieved 2009-03-31.
- ↑ Nature. 2002 Jan 24;415(6870):389-95.