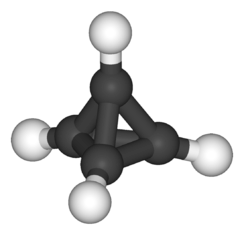
Tetrahedrane
 | |
| Names | |
|---|---|
| Preferred IUPAC name
Tetrahedrane | |
| Systematic IUPAC name
Tricyclo[1.1.0.02,4]butane | |
| Identifiers | |
| 3D model (Jmol) | Interactive image |
| 2035811 | |
| ChEBI | CHEBI:36549 |
| ChemSpider | 7827619 |
| PubChem | 9548696 |
| |
| |
| Properties | |
| C4H4 | |
| Molar mass | 52.08 g·mol−1 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Tetrahedrane is a platonic hydrocarbon with chemical formula C4H4 and a tetrahedral structure. Extreme angle strain (carbon bond angles deviate considerably from the tetrahedral bond angle of 109.5°) prevents this molecule from forming naturally.
Organic tetrahedranes
In 1978, Günther Maier prepared a stable tetrahedrane derivative with four tert-butyl substituents.[1] These substituents are very bulky, and completely envelop the tetrahedrane core. Maier suggested that bonds in the core are prevented from breaking because this would force the substituents closer together (corset effect) resulting in Van der Waals strain. Tetrahedrane is one of the possible platonic hydrocarbons and has the IUPAC name tricyclo[1.1.0.02,4]butane.
Unsubstituted tetrahedrane (C4H4) remains elusive, although it is predicted to be kinetically stable. One strategy that has been explored (but thus far failed) is reaction of propene with atomic carbon.[2] Locking away a tetrahedrane molecule inside a fullerene has only been attempted in silico.[3]
Tetra-tert-butyltetrahedrane
The tert-butyl derivative was first synthesised starting from a cycloaddition of an alkyne with t-Bu substituted maleic anhydride,[4] followed by rearrangement with carbon dioxide expulsion to a cyclopentadienone and its bromination, followed by addition of the fourth t-Bu group and a photochemical rearrangement with expulsion of carbon monoxide.
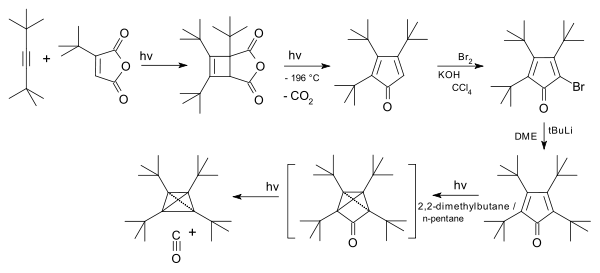 Tetra-tert-butyl-tetrahedrane synthesis 1978
Tetra-tert-butyl-tetrahedrane synthesis 1978
Tetra(trimethylsilyl)tetrahedrane
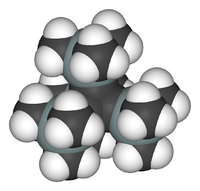
In tetra(trimethylsilyl)tetrahedrane (I) the tert-butyl groups have been replaced by trimethylsilyl groups.[5] This compound (prepared from the corresponding cyclobutadiene) is far more stable than the tert-butyl analogue. The silicon–carbon bond is longer than a carbon–carbon bond, and therefore the corset effect is reduced. On the other hand, the trimethylsilyl group is a σ-donor which explains the increased stabilization of the tetrahedrane. Whereas the tert-butyl tetrahedrane melts at 135 °C, at which temperature decomposition to the cyclobutadiene starts, the trimethylsilyl tetrahedrane melts at a much higher temperature of 202 °C, and is stable up to 300 °C, at which point it reverts to the acetylene starting material. The tetrahedrane skeleton is made up of banana bonds, and hence the carbon atoms are high in s-orbital character. From NMR, sp-hybridization can be deduced, normally reserved for triple bonds. As a consequence the bond lengths are unusually short with 152 picometers.
An improved synthesis of tetra(trimethylsilyl)tetrahedrane has been reported, by one-electron reduction of the cyclobutadiene precursor with tris(pentafluorophenyl)borane.[6] Reaction with methyllithium has yielded the stable tetrahedranyllithium derivative.[7] Coupling reactions with this lithium compound have given access to more derivatives.[8][9][10]
The tetrahedrane dimer (II) has also been reported.[11] The connecting bond is even shorter with 143.6 pm. An ordinary carbon–carbon bond has a length of 154 pm.
 Synthesis of tetra(trimethylsilyl)tetrahedrane and the dimer compound
Synthesis of tetra(trimethylsilyl)tetrahedrane and the dimer compound
Tetrasilatetrahedrane
In tetrasilatetrahedrane, the carbon atoms in the tetrahedrane cage are replaced by silicon. The standard silicon–silicon bond is much longer (235 pm) and the cage is again enveloped by a total of 16 trimethylsilyl groups. This makes the compound thermally stable. The silatetrahedrane can be reduced with potassium graphite to the tetrasilatetrahedranide potassium salt. In this compound one of the silicon atoms of the cage has lost a silyl substituent and carries a negative charge. The potassium cation can be captured by a crown ether and in the resulting complex potassium and the silyl anion are separated by a distance of 885 pm. One of the Si−–Si bonds is now 272 pm and its silicon atom has an inverted tetrahedral geometry. Furthermore, the four cage silicon atoms are equivalent on the NMR timescale due to migrations of the silyl substituents over the cage.[12]
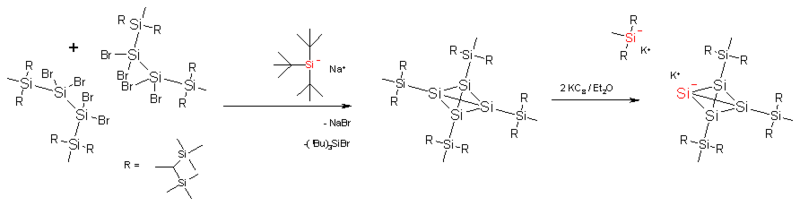 Tetrasilatetrahedrane
Tetrasilatetrahedrane
The dimerization reaction observed for the carbon tetrahedrane compound is also attempted for a tetrasilatetrahedrane.[13] In this tetrahedrane the cage is protected by four so-called supersilyl groups in which a silicon atom has 3 tert-butyl substituents. The dimer does not materialize but a reaction with iodine in benzene followed by reaction with the tri-tert-butylsilaanion results in the formation of an eight-membered silicon cluster compound which can be described as a Si2 dumbbell (length 229 pm and with inversion of tetrahedral geometry) sandwiched between two almost-parallel Si3 rings.
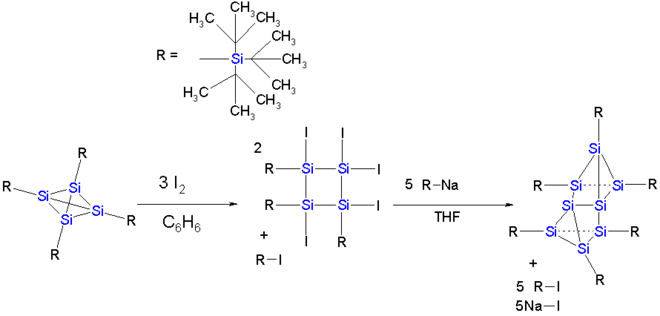 Silicon cluster compound
Silicon cluster compound
In known eight-membered clusters of in the same carbon group, tin Sn8R6 and germanium Ge8R6 the cluster atoms are located on the corners of a cube.
Tetranitrotetrahedrane
Due to its bond strain and perfect oxygen balance, tetranitrotetrahedrane, an analogue of tetrahedrane with four nitro group substituents, has potential as a high-performance energetic material. The addition of these nitro groups is likely to reduce tetranitrohedrane's stability.[14]
Inorganic and organometallic tetrahedranes

The tetrahedrane motif occurs broadly in chemistry. White phosphorus (P4) and yellow arsenic (As4) are examples. Several types of metal carbonyl clusters are referred to as tetrahedranes.
See also
References
- ↑ Maier, G.; Pfriem, S.; Schäfer, U.; Matusch, R. (1978). "Tetra-tert-butyltetrahedrane". Angew. Chem. Int. Ed. Engl. 17 (7): 520–521. doi:10.1002/anie.197805201.
- ↑ Nemirowski, Adelina; Reisenauer, Hans Peter; Schreiner, Peter R. (2006). "Tetrahedrane—Dossier of an Unknown". Chem. Eur. J. 12: 7411–7420. doi:10.1002/chem.200600451.
- ↑ Ren, Xiao-Yuan; Jiang, Cai-Ying; Wang, Jiang; Liu, Zi-Yang (2008). "Endohedral complex of fullerene C60 with tetrahedrane, C4H4@C60". J. Mol. Graph. Model. 27: 558–562. doi:10.1016/j.jmgm.2008.09.010.
- ↑ Maier, Günther; Boßlet, Friedrich (1972). "tert-Butyl-substituierte cyclobutadiene und cyclopentadienone" [tert-Butyl-substituted cyclobutadienes and cyclopentadienones]. Tetrahedron Letters. 13 (11): 1025–1030. doi:10.1016/S0040-4039(01)84500-7.
- ↑ Maier, Günther; Neudert, Jörg; Wolf, Oliver; Pappusch, Dirk; Sekiguchi, Akira; Tanaka, Masanobu; Matsuo, Tsukasa (2002). "Tetrakis(trimethylsilyl)tetrahedrane". J. Am. Chem. Soc. 124 (46): 13819–13826. doi:10.1021/ja020863n.
- ↑ Nakamoto, M.; Inagaki, Y.; Ochiai, T.; Tanaka, M.; Sekiguchi, A. (2011). "Cyclobutadiene to tetrahedrane: Valence isomerization induced by one-electron oxidation". Heteroatom. Chem. 22: 412–416. doi:10.1002/hc.20699.
- ↑ Sekiguchi, Akira; Tanaka, Masanobu (2003). "Tetrahedranyllithium: Synthesis, Characterization, and Reactivity". J. Am. Chem. Soc. 125 (42): 12684–12685. doi:10.1021/ja030476t.
- ↑ Nakamoto, Masaaki; Inagaki, Yusuke; Nishina, Motoaki; Sekiguchi, Akira (2009). "Perfluoroaryltetrahedranes: Tetrahedranes with Extended σ−π Conjugation". J. Am. Chem. Soc. 131 (9): 3172–3173. doi:10.1021/ja810055w.
- ↑ Ochiai, Tatsumi; Nakamoto, Masaaki; Inagaki, Yusuke; Sekiguchi, Akira (2011). "Sulfur-Substituted Tetrahedranes". J. Am. Chem. Soc. 133 (30): 11504–11507. doi:10.1021/ja205361a.
- ↑ Kobayashi, Y.; Nakamoto, M.; Inagaki, Y.; Sekiguchi, A. (2013). "Cross-Coupling Reaction of a Highly Strained Molecule: Synthesis of σ–π Conjugated Tetrahedranes". Angew. Chem. Int. Ed. 52 (41): 10740–10744. doi:10.1002/anie.201304770.
- ↑ Tanaka, M.; Sekiguchi, A. (2005). "Hexakis(trimethylsilyl)tetrahedranyltetrahedrane". Angew. Chem. Int. Ed. 44 (36): 5821–5823. doi:10.1002/anie.200501605. PMID 16041816.
- ↑ Ichinohe, Masaaki; Toyoshima, Masafumi; Kinjo, Rei; Sekiguchi, Akira (2003). "Tetrasilatetrahedranide: A Silicon Cage Anion". J. Am. Chem. Soc. (communication). 125 (44): 13328–13329. doi:10.1021/ja0305050. PMID 14583007.
- ↑ Fischer, G.; Huch, V.; Mayer, P.; Vasisht, S. K.; Veith, M.; Wiberg, N. (2005). "Si8(SitBu3)6: A Hitherto Unknown Cluster Structure in Silicon Chemistry". Angewandte Chemie International Edition. 44 (48): 7884–7887. doi:10.1002/anie.200501289. PMID 16287188.
- ↑ "Computational studies on a kind of novel energetic materials tetrahedrane and nitro derivatives". Journal of Molecular Structure: Theochem. 668: 189–195. doi:10.1016/j.theochem.2003.10.054.