trans-3-Methyl-4-octanolide
 | |
| Names | |
|---|---|
| IUPAC name
(4R,5S)-5-butyl-4-methyldihydrofuran-2(3H)-one | |
| Identifiers | |
| 3D model (Jmol) | (4R,5S): Interactive image (4R,5S): Interactive image |
| ChemSpider | 9280733 (4R,5S) 9259269 (4S,5R) |
| PubChem | 11105597 (4R,5S) 11084123 (4S,5R) |
| |
| |
| Properties | |
| C9H16O2 | |
| Molar mass | 156.222 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
trans-3-Methyl-4-octanolide, also called trans-β-methyl-γ-octalactone is a chemical compound of the lactone family with formula C
9H
16O
2. It exists in two stereoisomers: (3R,4S) and (3S,4R).
The (3S,4R) form is found in whiskey and other alcoholic beverages that have been aged in oak barrels, together with the more important cis isomer.[1][2] It has a coconut, celery or fresh wood aroma, that can be detected by humans at the concentration of 20 μg/L in air.[3] A mixture of the cis and trans isomers is repellent for mosquitos and flies.[4]
The (3S,4R) isomer is extracted by the alchoolic beverage from some precursor substances in the oak wood.[5] It can be synthesized in various ways.[3][6][7]
See also
- cis-3-Methyl-4-octanolide, the more important stereoisomer
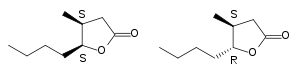 3-Methyl-4-octanolide, cis and trans isomers.
3-Methyl-4-octanolide, cis and trans isomers.
References
- ↑ "Aromas and Flavours". Wine-Pages.com. Retrieved 2007-12-18.
- ↑ Hans-Dieter Belitz, Peter Schieberle, and Werner Grosch (2004) Food Chemistry, page 383. Springer, ISBN 3-540-40818-5
- 1 2 Eric Masson, Raymond Baumes, Christine Le Guernevé, and Jean-Louis Puech (2000) Identification of a Precursor of β-Methyl-γ-octalactone in the Wood of Sessile Oak (Quercus petraea (Matt.) Liebl.). J. Agric. Food Chem. volume 48, pages 4306-4309
- ↑ Yukio Suzuki, Wakako Mori, Hiroyuki Ishizone, Koichi Naito, and Toshio Honda (1992) Concise Enantiospecific Syntheses of (+)-Eldanolide and (−)-cis-Whisky Lactone. Tetrahedron letters, volume 33, pages 4931-4932 doi:10.1016/S0040-4039(00)61237-6
- ↑ Masuda and Nishimura (1971), Branched nonalactones from some Quercus species. Phytochemistry, volume 10, pages 1401-1402.
- ↑ Hisashi Nishikori, Katsuji Ito, and Tsutomu Katsuki (1998) A short-step synthesis of trans-whisky lactone by an asymmetric Michael reaction. Tetrahedron: Asymmetry, volume 9, pages 1165–1170.
- ↑ Katsuji Ito, Miwa Yoshitake and Tsutomu Katsuki (1995), Enantioselective Synthesis of trans-Whisky Lactone by Using Newly Developed Asymmetric Ring Expansion Reaction of Oxetane as a Key Step. Chemistry Letters, volume 24, issue 11, page 1027 doi:10.1246/cl.1995.1027
This article is issued from Wikipedia - version of the 10/29/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.