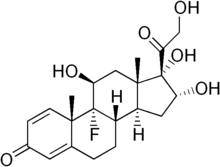
Triamcinolone
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Kenalog Nasacort |
| AHFS/Drugs.com | Monograph |
| Pregnancy category | |
| Routes of administration | Oral, topical, IM, intra-articular, intrasynovial |
| ATC code | A01AC01 (WHO) C05AA12 (WHO), D07AB09 (WHO), H02AB08 (WHO), R01AD11 (WHO), R03BA06 (WHO), S01BA05 (WHO) |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 68% |
| Metabolism | Hepatic |
| Biological half-life | 88 minutes |
| Excretion | Fecal and renal |
| Identifiers | |
| |
| Synonyms |
Click show to see
(8S,9R,10S,11S,13S,14S,16R,17S)-9-fluoro-11,16,17-trihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-6,7,8,9,10,11,12,13,14,15,16,17-dodecahydro-3H-cyclopenta[a]phenanthren-3-one; (1R,2S,10S,11S,13R,14S,15S,17S)-1-fluoro-13,14,17-trihydroxy-14-(2-hydroxyacetyl)-2,15-dimethyltetracyclo[8.7.0.02,7.011,15]heptadeca-3,6-dien-5-one |
| CAS Number |
124-94-7 |
| PubChem (CID) | 31307 |
| IUPHAR/BPS | 2870 |
| DrugBank |
DB00620 |
| ChemSpider |
29046 |
| UNII |
1ZK20VI6TY |
| KEGG |
D00385 |
| ChEBI |
CHEBI:9667 |
| ChEMBL |
CHEMBL1451 |
| ECHA InfoCard | 100.004.290 |
| Chemical and physical data | |
| Formula | C21H27FO6 |
| Molar mass | 394.434 g/mol |
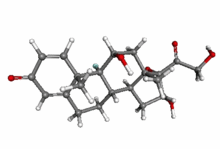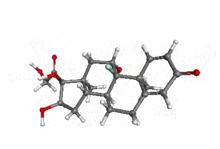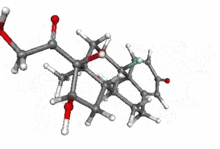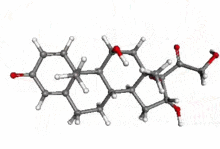
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Triamcinolone is a long-acting synthetic corticosteroid given orally, by injection, by inhalation, or as a topical ointment or cream.
Uses
Triamcinolone is used to treat a number of different medical conditions, such as eczema, psoriasis, arthritis, allergies, ulcerative colitis, lupus, sympathetic ophthalmia, temporal arteritis, uveitis, ocular inflammation, Urushiol-induced contact dermatitis, aphthous ulcers (usually as triamcinolone acetonide), visualization during vitrectomy and the prevention of asthma attacks. It will not treat an asthma attack once it has already begun.[1][2][3] It has also been used off-label for macular degeneration.[4]
Prior to 2007 it was sold under the name Azmacort as a corticosteroid inhaler for asthma long-term care.
In 2010, TEVA and Perrigo launched the first generic inhalable triamcinolone.[5]
Triamcinolone is used to alleviate infection-induced eczema in fungal skin infections in the combination drug of econazole/triamcinolone.
The derivative triamcinolone acetonide is one of the ingredients of Ledermix, an endodontic (tooth's root canal) lotion used between sessions, and Sanofi sold it under the brand name Nasacort. Triamcinolone acetonide is also used as intra lesional steroid injection to treat keloids and hypertrophic scars.
According to Chang et al (2014), "Triamcinolone acetonide (TA) is classified as an S9 glucocorticoid in the 2014 Prohibited List published by the World Anti-Doping Agency, which caused it to be prohibited in-competition when administered orally, intravenously, intramuscularly or rectally".[6]
Forms
Different triamcinolone derivatives are available, including acetonide, benetonide, furetonide, hexacetonide and diacetate.
Triamcinolone acetonide is a more potent type of triamcinolone, being about eight times as effective as prednisone.
Side effects
Side effects of triamcinolone include sore throat, nosebleeds, increased coughing, headache, and runny nose. White patches in the throat or nose indicate a serious side effect. Symptoms of an allergic reaction include rash, itch, swelling, severe dizziness, trouble breathing.[7] An additional side effect for women is a prolonged menstrual cycle.
Trade names
Trade names for triamcinolone include Aristocort (Sandoz, now Novartis), Kenacort (Bristol-Myers Squibb), Kenalog (Bristol-Myers Squibb), Tricort (Cadila), Triaderm (Schering-Plough), Azmacort (KOS), Trilone, Volon A, Tristoject, Tricortone and Ratio-Triacomb.
See also
- Glucocorticoid (a chart comparing various glucocorticoids)
- Triamcinolone acetonide
References
- ↑ Triamcinolone - Drugs.com
- ↑ Triamcinolone Inhalation - Drugs.com
- ↑ Alcon Receives FDA Approval of Triesence Injectable Triamcinolone Suspension for Use in Eye Surgery - Drugs.com
- ↑ Age-Related Macular Degeneration (AMD) Treatment
- ↑ Perrigo Announces Launch Of Generic Version Of Nasacort AQ - CBS Detroit
- ↑ Chang, Chih-Wei; Huang, Tai-Yuan; Tseng, Yi-Chun; Chang-Chien, Guo-Ping; Lin, Su-Fan; Hsu, Mei-Chich (2014). "Positive doping results caused by the single-dose local injection of triamcinolone acetonide". Forensic Science International. 244: 1–6. doi:10.1016/j.forsciint.2014.07.024. PMID 25126738.
- ↑ "Drugs and Treatments - Nasacort AQ Nasl - Patient Handout". WebMD. Retrieved 2008-03-24.