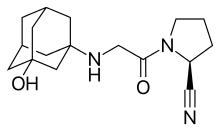
Vildagliptin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Galvus |
| AHFS/Drugs.com | International Drug Names |
| License data | |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code |
A10BH02 (WHO) A10BD08 (WHO) (with metformin)[1] |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 85% |
| Protein binding | 9.3% |
| Metabolism | Mainly hydrolysis to inactive metabolite; CYP450 not appreciably involved |
| Biological half-life | 2 to 3 hours |
| Excretion | Renal |
| Identifiers | |
| |
| Synonyms | (2S)-1-{2-[(3-hydroxy-1-adamantyl)amino]acetyl}pyrrolidine-2-carbonitrile |
| CAS Number |
274901-16-5 |
| PubChem (CID) | 6918537 |
| IUPHAR/BPS | 6310 |
| DrugBank |
DB04876 |
| ChemSpider |
5293734 |
| UNII |
I6B4B2U96P |
| KEGG |
D07080 |
| ChEMBL |
CHEMBL142703 |
| ECHA InfoCard | 100.158.712 |
| Chemical and physical data | |
| Formula | C17H25N3O2 |
| Molar mass | 303.399 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| | |
Vildagliptin (previously LAF237, trade names Galvus, Zomelis,) is an oral anti-hyperglycemic agent (anti-diabetic drug) of the new dipeptidyl peptidase-4 (DPP-4) inhibitor class of drugs. Vildagliptin inhibits the inactivation of GLP-1[2][3] and GIP[3] by DPP-4, allowing GLP-1 and GIP to potentiate the secretion of insulin in the beta cells and suppress glucagon release by the alpha cells of the islets of Langerhans in the pancreas.
Vildagliptin has been shown to reduce hyperglycemia in type 2 diabetes mellitus.[2]
Combination with metformin
The EMEA has also approved a new oral treatment released by Novartis, called Eucreas, a combination of vildagliptin and metformin.[4]
Adverse Effects
Adverse effects observed in clinical trials include nausea, hypoglycemia, tremor, headache and dizziness. Rare cases of hepatoxicity have been reported.[5]
There have been case reports of pancreatitis associated with DPP-IV inhibitors. A group at UCLA reported increased pre-cancerous pancreatic changes in rats and in human organ donors who had been treated with DPP-IV inhibitors.[6][7] In response to these reports, the United States FDA and the European Medicines Agency each undertook independent reviews of all clinical and preclinical data related to the possible association of DPP-IV inhibitors with pancreatic cancer. In a joint letter to the New England Journal of Medicines, the agencies stated that "Both agencies agree that assertions concerning a causal association between incretin-based drugs and pancreatitis or pancreatic cancer, as expressed recently in the scientific literature and in the media, are inconsistent with the current data. The FDA and the EMA have not reached a final conclusion at this time regarding such a causal relationship. Although the totality of the data that have been reviewed provides reassurance, pancreatitis will continue to be considered a risk associated with these drugs until more data are available; both agencies continue to investigate this safety signal."[8]
See also
References
- ↑ WHO International Working Group for Drug Statistics Methodology (August 27, 2008). "ATC/DDD Classification (FINAL): New ATC 5th level codes". WHO Collaborating Centre for Drug Statistics Methodology. Retrieved 2008-09-05.
- 1 2 Ahrén, B; Landin-Olsson, M; Jansson, PA; Svensson, M; Holmes, D; Schweizer, A (2004). "Inhibition of dipeptidyl peptidase-4 reduces glycemia, sustains insulin levels, and reduces glucagon levels in type 2 diabetes". The Journal of Clinical Endocrinology and Metabolism. 89 (5): 2078–84. doi:10.1210/jc.2003-031907. PMID 15126524.
- 1 2 Mentlein, R; Gallwitz, B; Schmidt, WE (1993). "Dipeptidyl-peptidase IV hydrolyses gastric inhibitory polypeptide, glucagon-like peptide-1(7-36)amide, peptide histidine methionine and is responsible for their degradation in human serum". European Journal of Biochemistry / FEBS. 214 (3): 829–35. doi:10.1111/j.1432-1033.1993.tb17986.x. PMID 8100523.
- ↑ EU approves Novartis's Eucreas diabetes drug. Reuters, February 25, 2008.
- ↑ "www.ema.europa.eu" (PDF).
- ↑ Matveyenko AV, Dry S, Cox HI, et al. (July 2009). "Beneficial endocrine but adverse exocrine effects of sitagliptin in the human islet amyloid polypeptide transgenic rat model of type 2 diabetes: interactions with metformin". Diabetes. 58 (7): 1604–15. doi:10.2337/db09-0058. PMC 2699878
 . PMID 19403868.
. PMID 19403868. - ↑ Butler AE, Campbell-Thompson M, Gurlo T, Dawson DW, Atkinson M, Butler PC (July 2013). "Marked expansion of exocrine and endocrine pancreas with incretin therapy in humans with increased exocrine pancreas dysplasia and the potential for glucagon-producing neuroendocrine tumors". Diabetes. 62 (7): 2595–604. doi:10.2337/db12-1686. PMC 3712065
 . PMID 23524641.
. PMID 23524641. - ↑ Egan, Amy G.; Blind, Eberhard; Dunder, Kristina; De Graeff, Pieter A.; Hummer, B. Timothy; Bourcier, Todd; Rosebraugh, Curtis (2014). "Pancreatic Safety of Incretin-Based Drugs — FDA and EMA Assessment — NEJM". New England Journal of Medicine. 370 (9): 794–7. doi:10.1056/NEJMp1314078. PMID 24571751.
External links
- Banting and Best Diabetes Centre at UT vildagliptin – Vildagliptin
- Banting and Best Diabetes Centre at UT dpp4 – About DPP-4
- The race to get DPP-4 inhibitors to market – Forbes.com
- Merck's March Madness – Forbes.com