2- and 4-Quinolones

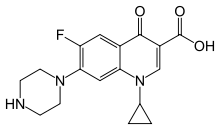
The 2- and 4-quinolones are a class of bicyclic molecules, organic chemical structures that are related to the heteroaromatic coal tar isolate quinoline, see image at right. Specific quinoline molecules substituted with an hydroxyl functional group at carbons 2 and 4 (C-2 and C-4, see image) are most often observed in isomeric forms termed 2- and 4-quinolones, respectively (tautomers, relationships explained further below). The relative importance of 4-quinolones has increased with the discovery that such structures that also bear a carboxylic acid (-COOH) and other functional groups at particular sites on the ring have very potent bacteriocidal activities, inhibiting of a broad spectrum of Gram negative and Gram positive DNA gyrase and topoisomerase enzymes. Hence, they are very useful in antibacterial therapy. An example of such a 4-quinolone is "cipro" (ciprofloxacin, image at right), where the atoms of quinoline can be traced within this related structure. Ciprofloxacin is a "second-generation" fluoroquinolone antibacterial (see below), introduced by Bayer AG and still in wide use as the second decade of the new millennium begins.
Relationship, hydroxyquinolines and quinolones
In the chemical synthesis of specific quinolines, the nitrogen-containing ring is often "closed" as a part of the synthetic process, and in some syntheses, doing so can install a hydroxyl group (an –OH functional group) on carbons adjacent to or across from the ring nitrogen (i.e., the C-2 and the C-4 positions, see quinoline structure above). An example of such a synthesis is the Camps cyclization, which, depending on exact starting materials and reaction conditions, can give both 2-hydroxyquinolines (B) and 4-hydroxyquinolines (A) as shown.
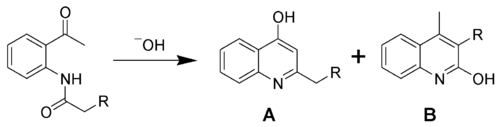

.

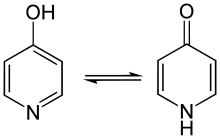
Such hydroxy-substituted quinolines are, most often, not distinguished from a particular isomeric form termed a tautomer (see Prof. Reusch's further definition in the Notes).[1] In this particular rapid isomerization, the labile proton (H+) migrates from the hydroxyl group to the ring nitrogen, and the double bond (pi-electron density) migrates from within the ring to the carbon-oxygen bond to form a carbonyl group. These tautomerizations are represented in the images at right by the same process in closely related structures. (The tautomerizations also take place when the adjacent all-carbon ring is absent.) When represented as the carbonyl-containing tautomer—often the most stable form, so the one observed in structure measurements such as room temperature NMR—the pair of hydroxy-substitituted quinolines shown above are formally referred to as 2-quinolones (oxygen adjacent to the ring N) or 4-quinolones (oxygen across from the ring N), as the case may be. Hence, ciprofloxacin (shown above) is 4-quinolone.
Quinolones as antibacterials
4-Quinolones are a family of synthetic broad-spectrum agents used as anti-bacterial agents.[2] The first generation of the quinolones began following introduction of the related, but structurally distinct naphthyridine-family nalidixic acid in 1962 for treatment of urinary tract infections in humans.[3] Nalidixic acid was discovered by George Lesher and coworkers in a chemical distillate during an attempt at synthesis of the chloroquinoline antimalarial agent, chloroquine.[4] Naphthyridone and quinolone classes of antibiotics prevent bacterial DNA replication by inhibition of DNA unwinding events, and can be both bacteriostatic and bacteriocidal.[5] (See Mechanism of Action later.) The majority of quinolones in clinical use belong to the second generation class of "fluoroquinolones", which have a true quinoline framework, maintain the C-3 carboxylic acid group, and add a fluorine atom to the all-carbon containing ring, typically at the C-6 or C-7 positions.
History
Though it is generally accepted that 1,8-naphthyridine-type agent nalidixic acid, image at right, is the predecessor of quinolone antibacterials, the matter has been contested by a few researchers who promote chloroquine, from which nalidixic acid was derived, as the forerunner of the quinolones. As a predecessor, the nalidixic acid molecular shape foreshadowed the entire, extensive quinolone family, which includes second, third, and fourth generation quinolones, and further agents under development (next section). This first, nalidixic acid generation included other such "quinolone" drugs as oxolinic acid (a true 4-quinolone), pipemidic acid (a pyridopyrimidine), and cinoxacin (a cinnoline), all introduced in the 1970s. Each proved to be only marginal improvements over nalidixic acid.[6] Since the introduction of nalidixic acid in 1962, more than 10,000 quinolone analogs have been synthesized; and as is standard in modern medicinal chemistry, a handful have found their way into wide clinical practice.[7]
Generations
Researchers divide the quinolones into generations based on their antibacterial spectrum.[8][9] The earlier-generation agents are, in general, more narrow-spectrum than the later ones, but there is no standard employed to determine which drug belongs to which generation. The only universal standard applied is the grouping of the non-fluorinated drugs found within this class (quinolones) within the first-generation heading. As such, there exists a wide variation within the literature dependent upon the methods employed by the authors.
- Some researchers group these drugs by patent dates
- Some by a specific decade (i.e., '60s, '70s, '80s, etc.)
- Others by the various structural changes
The first generation is rarely used today. The drugs most frequently prescribed today consist of Avelox (moxifloxacin), Cipro (ciprofloxacin), Levaquin (levofloxacin), and, to some extent, their generic equivalents.
First-generation.
- cinoxacin (Cinobac) [10]
- flumequine (Flubactin) (veterinary use)
- nalidixic acid (NegGam, Wintomylon)[10]
- oxolinic acid (Uroxin) (currently unavailable in the United States)
- piromidic acid (Panacid) (currently unavailable in the United States)
- pipemidic acid (Dolcol) (currently unavailable in the United States)
- rosoxacin (Eradacil)
Second-generation. The second-generation class is sometimes subdivided into "Class 1" and "Class 2".[11]
- ciprofloxacin (Zoxan, Ciprobay, Cipro, Ciproxin)[10][12]
- enoxacin (Enroxil, Penetrex)[10]
- fleroxacin (Megalone, Roquinol)
- lomefloxacin (Maxaquin)[10]
- nadifloxacin (Acuatim, Nadoxin, Nadixa) (currently unavailable in the United States)
- norfloxacin (Lexinor, Noroxin, Quinabic, Janacin)
- ofloxacin (Floxin, Oxaldin, Tarivid)[10] (only as ophthalmic in the United States)
- pefloxacin (Peflacine) (currently unavailable in the United States)
- rufloxacin (Uroflox) (currently unavailable in the United States)
Third-generation. Unlike the first- and second-generations, the third-generation is active against streptococci.[11]
- balofloxacin (Baloxin) (currently unavailable in the United States)
- grepafloxacin (Raxar)
- levofloxacin (Cravit, Levaquin)[10][12]
- pazufloxacin (Pasil, Pazucross) (currently unavailable in the United States)
- sparfloxacin (Zagam)[10] (currently unavailable in the United States),[13]
- temafloxacin (Omniflox)
- tosufloxacin (Ozex, Tosacin) (currently unavailable in the United States)
Fourth-generation. Fourth generation fluoroquinolones act at DNA gyrase and topoisomerase IV.[14] This dual action slows development of resistance.
- clinafloxacin[12] (currently unavailable in the United States)
- gatifloxacin (Zigat, Tequin) (Zymar -opth.) (Tequin removed from clinical use)[15]
- gemifloxacin (Factive)
- moxifloxacin (Avelox,Vigamox)[10]
- sitafloxacin (Gracevit) (currently unavailable in the United States)
- trovafloxacin (Trovan) (removed from clinical use)[10][12]
- prulifloxacin (Quisnon) (currently unavailable in the United States)
In development.
Pharmacology
The basic pharmacophore, or active structure, of the fluoroquinolone class is based upon the quinoline ring system.[16] Various substitutions made to the quinoline ring resulted in the development of numerous fluoroquinolone drugs available today. The addition of the fluorine atom at C-6 distinguishes the successive-generation fluoroquinolones from the first-generation quinolones, though examples in early discovery stages of research are known that omit the atom while retaining antibacterial activity.[17]
Mechanism of action
Quinolones and fluoroquinolones are chemotherapeutic bactericidal drugs, eradicating bacteria by interfering with DNA replication. Quinolones inhibit the bacterial DNA gyrase or the topoisomerase II enzyme, thereby inhibiting DNA replication and transcription. Recent evidence has shown topoisomerase II is also a target for a variety of quinolone-based drugs. Thus far, most of the compounds that show high activity against the eukaryotic type II enzyme contain aromatic substituents at their C-7 positions.[18]
Quinolones can enter cells easily and, therefore, are often used to treat intracellular pathogens such as Legionella pneumophila and Mycoplasma pneumoniae. For many Gram-negative bacteria, DNA gyrase is the target, whereas topoisomerase IV is the target for many Gram-positive bacteria. It is believed eukaryotic cells do not contain DNA gyrase or topoisomerase IV. However, there is debate concerning whether the quinolones still have such an adverse effect on the DNA of healthy cells
Veterinary use
The quinolones have been widely used in animal husbandry, and several agents have veterinary-specific applications.
- danofloxacin (Advocin, Advocid) (for veterinary use)
- difloxacin (Dicural, Vetequinon) (for veterinary use)
- enrofloxacin (Baytril) (for veterinary use)
- ibafloxacin (Ibaflin) (for veterinary use)
- marbofloxacin (Marbocyl, Zenequin) (for veterinary use)
- orbifloxacin (Orbax, Victas) (for veterinary use)
- sarafloxacin (Floxasol, Saraflox, Sarafin) (for veterinary use)
See also
References
- ↑ Per Prof. Reusch (Michigan State), tautomers are "rapidly interconverted constitutional isomers, usually distinguished by a different bonding location for a labile hydrogen atom ... and a differently located double bond. See www2.chemistry.msu.edu:80/faculty/reusch/VirtTxtJml/react2.htm . Retrieved 13 April 2011.
- ↑ Ivanov DV, Budanov SV (2006). "[Ciprofloxacin and antibacterial therapy of respiratory tract infections]". Antibiot. Khimioter. (in Russian). 51 (5): 29–37. PMID 17310788.
- ↑ sanofi-aventis U.S. LLC (September 2008). "NegGram® Caplets (nalidixic acid, USP)" (PDF). USA: FDA. Retrieved 6 May 2011.
- ↑ Wentland MP: In memoriam: George Y. Lesher, PhD, in Hooper DC, Wolfson JS (eds): Quinolone antimicrobial agents, ed 2., Washington DC, American Society for Microbiology : XIII – XIV, 1993.
- ↑ Hooper, DC. (Mar–Apr 2001). "Emerging mechanisms of fluoroquinolone resistance." (PDF). Emerg Infect Dis. 7 (2): 337–41. doi:10.3201/eid0702.010239. PMC 2631735
 . PMID 11294736.
. PMID 11294736. - ↑ Norris, S; Mandell, GL (1988). "The quinolones: history and overview". The quinolones: history and overview. San Diego: Academic Press Inc. pp. 1–22.
- ↑ Stacy J. Childs, MD (2000). "Safety of the Fluoroquinolone Antibiotics: Focus on Molecular Structure". Infect Urol. USA: FQresearch. 13 (1): 3–10. Retrieved 6 May 2011.
- ↑ Ball P (2000). "Quinolone generations: natural history or natural selection?". J. Antimicrob. Chemother. 46 Suppl T1 (Supplement 3): 17–24. doi:10.1093/oxfordjournals.jac.a020889. PMID 10997595. Retrieved 6 May 2011.
- ↑ "New Classification and Update on the Quinolone Antibiotics – May 1, 2000 – American Academy of Family Physicians". Retrieved 18 March 2008.
- 1 2 3 4 5 6 7 8 9 10 "Quinolones: A Comprehensive Review – February 1, 2002 – American Family Physician". Archived from the original on 6 June 2011. Retrieved 6 May 2011.
- 1 2 Oliphant, CM; Green, GM (February 2002). "Quinolones: a comprehensive review". Am Fam Physician. 65 (3): 455–64. PMID 11858629. Archived from the original on 6 June 2011. Retrieved 6 May 2011.
- 1 2 3 4 Ambrose, Paul G.; Owens, Robert C., Jr (1 March 2000). "Clinical Usefulness Of Quinolones". Seminars in Respiratory and Critical Care Medicine. Medscape. Archived from the original on 8 June 2011. Retrieved 6 May 2011.
- ↑ UN (2005). "Consolidated list of products – Pharmaceuticals 12th issue" (PDF). United Nations. Retrieved 6 May 2011.
- ↑ Gupta (2009). Clinical Ophthalmology: Contemporary Perspectives, 9/e. Elsevier India. pp. 112–. ISBN 978-81-312-1680-4. Retrieved 20 September 2010.
- ↑ Schmid, Randolph E. (1 May 2006). "Drug Company Taking Tequin Off Market". Associated Press. Archived from the original on 25 November 2007. Retrieved 1 May 2006.
- ↑ Schaumann, R.; Rodloff, A. C. (January 2007). "Activities of Quinolones Against Obligately Anaerobic Bacteria" (PDF). Anti-Infective Agents in Medicinal Chemistry. Bentham Science Publishers. 6 (1): 49–56. doi:10.2174/187152107779314179. Archived (PDF) from the original on 7 June 2011. Retrieved 6 May 2011.
- ↑ Chang Y.H.; Se H.K.; Young K.K (22 July 1997). "Novel 5-amino-6-methylquinolone antibacterials: A new class of non-6-fluoroquinolones". Bioorganic & Medicinal Chemistry Letters. Elsevier. 7 (14): 1875–1878. doi:10.1016/S0960-894X(97)00324-7.
- ↑ Elsea, SH.; Osheroff, N.; Nitiss, JL. (July 1992). "Cytotoxicity of quinolones toward eukaryotic cells. Identification of topoisomerase II as the primary cellular target for the quinolone CP-115,953 in yeast." (PDF). J Biol Chem. 267 (19): 13150–3. PMID 1320012. Retrieved 6 May 2011.
External links
- 2- and 4-Quinolones at DMOZ
- Fact Sheet: Quinolones
- Structure Activity Relationships "Antibacterial Agents; Structure Activity Relationships," André Bryskier MD
