Desorption atmospheric pressure photoionization
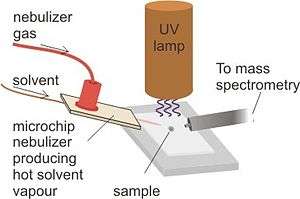
Desorption atmospheric pressure photoionization (DAPPI) is an ambient ionization technique for mass spectrometry that uses hot solvent vapor for desorption in conjunction with photoionization. DAPPI enables direct analysis of solid samples without pretreatment and analysis of samples deposited on surfaces by means of a jet of hot solvent vapour and vacuum ultraviolet light. The hot jet thermally desorbs the sample from a surface and the vaporized sample is ionized by the vacuum ultraviolet light and consequently sampled into a mass spectrometer.[1]
Principle of operation
Desorption process
Desorption of the sample is initiated by a hot jet of solvent vapor that is targeted onto the sample by a nebulizer microchip. The microchip is heated in order to vaporize the entering solvent and create dopant molecules in order to thermally desorb the sample surface.
Ionization
A beam of 10 eV photons that are given off by a UV lamp is directed at the newly desorbed molecules, as well as the dopant molecules. Photoionization then occurs, which knocks out the molecule's electron and produces an ion. Unfortunately, this technique alone is not highly efficient for different varieties of molecules, particularly those that are not easily protonated or deprotonated. In order to completely ionize samples, dopant molecules must help. the gaseous solvent can also undergo photoionization and act as an intermediate for ionization of the sample molecules. Once dopant ions are formed, proton transfer can occur with the sample, creating more sample ions. Once ions are formed, they are sent into a mass analyzer for manipulation and detection.
Desorption and ionization mechanisms
The main desorption mechanism in DAPPI is thermal desorption due to rapid heating of the surface. Therefore, DAPPI only works well for surfaces of low thermal conductivity. The ionization mechanism depends on the analyte and solvent used and for example the following analyte (M) ions may be formed: [M + H]+, [M - H]−, M+•, M−•.[2]
Types of component geometries
Reflection geometry
Considered the normal geometry of DAPPI, this mode is ideal for solid samples that do not need any former sample preparation. It is placed horizontally onto the plate and below the mass spec inlet. The UV lamp is directly above and releases photons to interact with the desorbed molecules formed.[2]
Transmission geometry
This mode is specialized for analyzing liquid samples, with a metal or polymer mesh replacing the sample plate in reflection geometry. The mesh is oriented 180 degrees from the nebulizer microchip and the mass spec inlet, with the lamp directing photons to the area where the mesh releases newly desorbed molecules.[2]
Applications
DAPPI can analyze both polar (e.g. verapamil) and nonpolar (e.g. anthracene) compounds. Performance of DAPPI has also been demonstrated on direct analysis of illicit drugs.[3]
See also
References
- ↑ Haapala M, Pól J, Saarela V, Arvola V, Kotiaho T, Ketola RA, Franssila S, Kauppila TJ, Kostiainen R (2007). "Desorption Atmospheric Pressure Photoionization". Anal. Chem. 79 (20): 7867–7872. doi:10.1021/ac071152g. PMID 17803282.
- 1 2 3 Luosujärvi L, Arvola V, Haapala M, Pól J, Saarela V, Franssila S, Kotiaho T, Kostiainen R, Kauppila TJ (2008). "Desorption and Ionization Mechanisms in Desorption Atmospheric Pressure Photoionization". Anal. Chem. 80 (19): 7460–7466. doi:10.1021/ac801186x. PMID 18778037.
- ↑ Kauppila TJ, Arvola V, Haapala M, Pól J, Aalberg L, Saarela V, Franssila S, Kotiaho T, Kostiainen R (2008). "Direct analysis of illicit drugs by desorption atmospheric pressure photoionization". Rapid Commun. Mass Spectrom. 22 (7): 979–985. doi:10.1002/rcm.3461. PMID 18320545.