Dichlorine heptoxide
 | |
 | |
| Names | |
|---|---|
| IUPAC name
Dichlorine heptoxide | |
| Other names
Chlorine(VII) oxide; Perchloric anhydride; (Perchloryloxy)chlorane trioxide | |
| Identifiers | |
| 10294-48-1 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:52356 |
| ChemSpider | 109884 |
| PubChem | 123272 |
| |
| |
| Properties | |
| Cl2O7 | |
| Molar mass | 182.901 g/mol |
| Appearance | colorless oil |
| Density | 1900 kg m−3 |
| Melting point | −91.57 °C (−132.83 °F; 181.58 K) |
| Boiling point | 82 °C (180 °F; 355 K) |
| Hazards | |
| Main hazards | oxidizer, contact explosive[1] |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Dichlorine heptoxide is the chemical compound with the formula Cl2O7. This chlorine oxide is the anhydride of perchloric acid. It is produced by the careful distillation of perchloric acid in the presence of the dehydrating agent phosphorus pentoxide:[1]
- 2 HClO4 + P4O10 → Cl2O7 + H2P4O11
The chlorine(VII) oxide can be distilled off from the mixture.
It may also be formed by illumination on mixtures of chlorine and ozone.[2] It slowly hydrolyzes back to perchloric acid, which is also hazardous when anhydrous.
Structure
Cl2O7 is an endothermic molecule, meaning it is intrinsically unstable.
- 2 Cl2O7 → 2 Cl2 + 7 O2 (ΔH = −135 kJ/mol)
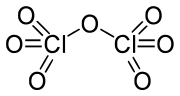
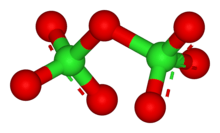
Cl2O7 is bent with Cl-O-Cl angle of 118.6° giving the molecule C2 symmetry. The terminal Cl-O distances are 1.709 Å and the Cl=O distances are 1.405 Å.[1] In this compound, chlorine exists in its highest formal oxidation state of +7, although the bonding in this molecule is significantly covalent.
Chemistry
Dichlorine heptoxide reacts with primary and secondary amines in carbon tetrachloride solution to yield N-perchloryls:[3]
- 2 RNH
2 + Cl
2O
7 → 2 RNHClO
3 + H
2O - 2 R
2NH + Cl
2O
7 → 2 R
2NClO
3 + H
2O
It also reacts with alkenes to give alkyl perchlorates. For example, it reacts with propene in carbon tetrachloride solution to yield isopropyl perchlorate and 1-chloro-2-propyl perchlorate.[4]
Dichlorine heptoxide is a strongly acidic oxide, and in solution it forms an equilibrium with perchloric acid.
Safety
Although it is the most stable chlorine oxide, Cl2O7 is a strong oxidizer as well as an explosive that can be set off with flame or mechanical shock, or by contact with iodine.[5] Nevertheless, it is less strongly oxidising than the other chlorine oxides, and does not attack sulfur, phosphorus, or paper when cold.[1] It has the same effects on the human body as elemental chlorine, and requires the same precautions.[6]
References
- 1 2 3 4 Holleman, Arnold F.; Wiberg, Egon (2001). Inorganic chemistry. Translated by Mary Eagleson; William Brewer. San Diego: Academic Press. p. 464. ISBN 0-12-352651-5.
- ↑ Byrns, A. C.; Rollefson, G. K. (1934). "The Formation of Chlorine Heptoxide on Illumination of Mixtures of Chlorine and Ozone". Journal of the American Chemical Society. 56 (5): 1250–1251. doi:10.1021/ja01320a506.
- ↑ Beard, C. D.; Baum, K. (1974). "Reactions of dichlorine heptoxide with amines". Journal of the American Chemical Society. 96 (10): 3237–3239. doi:10.1021/ja00817a034.
- ↑ Baum, K. . (1976). "Reactions of dichlorine heptoxide with olefins". The Journal of Organic Chemistry. 41 (9): 1663–1665. doi:10.1021/jo00871a048.
- ↑ Lewis, Robert Alan (1998). Lewis' dictionary of toxicology. CRC Press. p. 260. ISBN 1-56670-223-2.
- ↑ Jeanne Mager Stellman, ed. (1998). "Halogens and their compounds". Encyclopaedia of occupational health and safety (4th ed.). International Labour Organization. p. 104.210. ISBN 92-2-109817-6.