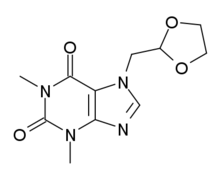
Doxofylline
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | R03DA11 (WHO) |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
69975-86-6 |
| PubChem (CID) | 50942 |
| DrugBank | DB09273 |
| ChemSpider | 46175 |
| UNII |
MPM23GMO7Z |
| KEGG |
D03898 |
| ECHA InfoCard | 100.067.468 |
| Chemical and physical data | |
| Formula | C11H14N4O4 |
| Molar mass | 266.25 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Doxofylline (also known as doxophylline) is a xanthine derivative drug used in the treatment of asthma.[1]
It has antitussive and bronchodilator[2] effects, and acts as a phosphodiesterase inhibitor.[3]
In animal and human studies, it has shown similar efficacy to theophylline but with significantly fewer side effects.[4]
Unlike other xanthines, doxofylline lacks any significant affinity for adenosine receptors and does not produce stimulant effects. This suggests that its antiasthmatic effects are mediated by another mechanism, perhaps its actions on phosphodiesterase.[1]
Doxofylline is available under the trade name Filodox in Bangladesh
References
- 1 2 Cirillo R, Barone D, Franzone JS (1988). "Doxofylline, an antiasthmatic drug lacking affinity for adenosine receptors". Arch Int Pharmacodyn Ther. 295: 221–37. PMID 3245738.
- ↑ Poggi R, Brandolese R, Bernasconi M, Manzin E, Rossi A (October 1989). "Doxofylline and respiratory mechanics. Short-term effects in mechanically ventilated patients with airflow obstruction and respiratory failure". Chest. 96 (4): 772–8. doi:10.1378/chest.96.4.772. PMID 2791671.
- ↑ Dini FL, Cogo R (2001). "Doxofylline: a new generation xanthine bronchodilator devoid of major cardiovascular adverse effects". Curr Med Res Opin. 16 (4): 258–68. doi:10.1185/030079901750120196. PMID 11268710.
- ↑ Sankar J, Lodha R, Kabra SK (March 2008). "Doxofylline: The next generation methylxanthine". Indian J Pediatr. 75 (3): 251–4. doi:10.1007/s12098-008-0054-1. PMID 18376093.
Further reading
- Shukla, Dali; Chakraborty, Subhashis; Singh, Sanjay; Mishra, Brahmeshwar (2009). "Doxofylline: A promising methylxanthine derivative for the treatment of asthma and chronic obstructive pulmonary disease". Expert Opinion on Pharmacotherapy. 10 (14): 2343. doi:10.1517/14656560903200667. PMID 19678793.
This article is issued from Wikipedia - version of the 7/17/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.