JNJ-7925476
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
129540-12-1 HCl: 109085-56-5 |
| PubChem (CID) | 13903191 |
| ChemSpider | 23130640 |
| UNII | VSM44B5G3G |
| ChEMBL | CHEMBL286312 |
| Chemical and physical data | |
| Formula | C20H19N |
| Molar mass | 273.371 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
JNJ-7925476 is a TRI antidepressant currently under development by Johnson & Johnson.[1]
These molecules were first prepared by Bruce E. Maryanoff, et al. during the late 1970s – 1980's.[2][3][4] and is known as a pyrroloisoquinoline, that is benzhydryl-containing.
Incorporating the pyrrolidino ring onto the THIQ scaffolding markedly improves potency, although this only works for one of the available diastereo/enantiomers. JNJ-7925476 is a racemic preparation of the more potent diastereomer. The eutomer, JNJ-39836966 has (6R,10bS)-stereochemistry whereas the distomer JNJ-39836732 will have (6S,10bR)-stereochemistry.
The compounds depicted appear to be cis but Maryanoff and coworkers are of the opinion that it is trans.[1] (see abstract)
The reason for this is not known because it was referred to as "cis" to begin with, only reassigning it later.
In-vitro characterization
Ki values (nM) for JNJ-7925476 and its constituent enantiomers (JNJ-39836966 and JNJ-39836732)
| JNJ | rSERT | hSERT | hDAT | hNET |
|---|---|---|---|---|
| 7925476 | 0.4 | 0.9 | 5.2 | 16.6 |
| 39836966 | 0.33 | 0.27 | 1.6 | 15.8 |
| 39836732 | 17.0 | 4.1 | 74.3 | 227 |
In vitro, JNJ-7925476 is ~18-fold selective for the hSERT (0.9 nM) over the hNET (16.6 nM).
Ex vivo transporter occupancy of JNJ-7925476 (in rat brain) followed the ordering priority: NET > SERT > DAT.
This is consistent with the results cited earlier for rat brains (see SAR table dated 1987).
However, there is relatively poor correlation between the in vitro data presented for rats brains vs what was reported at the human transporters.
μ-Dialysis
Elevations in extracellular DA in vivo was higher than expected on the basis of the in vitro transporter affinities.
The authors speculate that this could be because in the PFC where DATs are low in number, DA is predominantly transported via the NET.[5]
- ~ 1 mg/kg of JNJ-7925476 caused concentrations of NE, 5-HT and DA to all be elevated by just under 500%, respectively.
Ex vivo occupancy of the DAT was much lower (<50%) at this dose though, whereas the NET and SERT were similar (~90%).
It took a much higher dose (c.f. 10 mg/kg) for the DAT occupancy to approach the same as the NET and SERT (i.e. saturation).
At saturation, the elevation in synaptic DA was extremely prolific (15 × baseline), whereas SER and NE was ≈ ½ this amount (i.e. 750%).
Pyrroloisoquinolines structure activity relationships
_with_tube_model.png)

| X | Y | V | W | MA (mg/kg) | ptosis (mg/kg) | DA (nM) | NE (nM) | 5-HT (nM) |
| H | H | H | H | 0.34 (0.59) | 0.07 (0.05) | 11.3 (4.4) | 0.60 (0.37) | 23.5 (12.4) |
| H | H | OMe | OMe | 15.1 | 3.8 | 15.0 | 53.7 | 1540 |
| H | H | OH | OH | 0.87 | 0.53 | 43.5 | 10.5 | 124 |
| OMe | H | H | H | 0.27 | 0.03 | 5.2 | 0.79 | 1.7 |
| OH | H | H | H | 0.40 | 0.09 | 5.1 | 0.74 | 3.2 |
| H | OMe | H | H | ~0.2 | 0.07 | 15.8 | 0.65 | 7.2 |
| H | OH | H | H | >10 | 0.11 | 10.1 | 0.85 | 24.6 |
| H | H | H | OMe | no data | no data | 2.8 | 2.2 | 4.5 |
| OMe | OMe | H | H | 2.0 | 0.13 | 71.9 | 3.4 | 18.1 |
| OH | OH | H | H | 0.19 | 0.11 | 10.1 | 0.81 | 33.1 |
| Cl | H | H | H | 0.55 | 0.34 | 1.7 | 0.16 | 1.5 |
| H | Cl | H | H | ~0.1 | <0.1 | 2.5 | 0.45 | 7.3 |
| Cl | H | H | Cl | 37.4 | ~4 | 3.2 | 3.2 | 2.9 |
| Cl | Cl | H | H | 0.39 | 0.14 | 0.99 | 0.68 | 1.8 |
| F | H | H | H | ~0.2 | ~0.2 | 8.4 | 1.4 | 8.5 |
| F | H | H | F | >30 | 0.05 | 7.7 | 0.55 | 4.4 |
| NH2 | H | H | H | ~0.2 | ~0.01 | 0.86 | 0.20 | 44 |
| SMe | H | H | H | >30 (no data) | 0.30 (no data) | 41.2 (23.5) | 3.0 (1.8) | 0.62 (0.39) |
| Ethynyl | H | H | H | ~0.5 | ~0.5 | 2.6 | 0.94 | 1.0 |
| diclofensine | 10.9 | 8.8 | 10.3 | |||||
| WIN-25978 | 7.2 | 41.1 | 879 | |||||
This is a collection of all of the analogs that had favorable biological activity or an interesting substitution pattern.
All compounds are racemic preparations with the exception that brackets are for pure (+) enantiomer.
Ring size structure activity relationships
Expanding the ring size from pyrrolidino to piperidinyl resulted in compounds that were impotent, although contracting the ring size from 5 → 4 did not have negative repercussions on the resultant potency.
Chemistry
The N-acyliminium cyclization route; and the mandelic acid and styrene oxide route were employed for most of the target compounds.

The SS/RR diastereomers as the principle products if one follows the above steps.[6][7]
It is possible to epimerize the product to the desired RS/SR diastereomers, but the equilibrium is only 50/50.
Hence, alternative synthetic methods needed to be sought to obtain the desired isomer/s in diastereochemical excess.
If instead of an "aryl" group, a tert-butyl or a cyclohexyl was used, then it was possible to alter the stereochemical discourse of the reaction.[8]
Stereoselective reaction
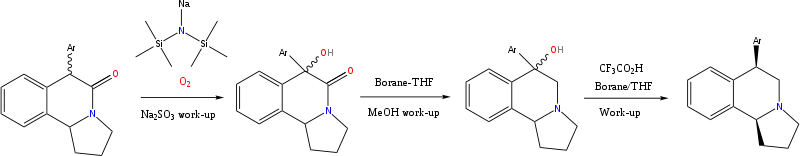
Hydrogenation of an appropriately positioned olefin might be expected to work.[9][10]
But the ketone cannot be reduced to an alcohol because it is part of an amide.
Notes
- Altinicline another rare instance of ethynyl attached directly to aromatic ring. Also occurs in RTI phenyltropane series. Erlotinib also.
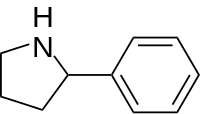
| 2,2-Diphenylethylamine:[11][12] | 2-Phenylpyrrolidine:[13][14] |
|---|---|
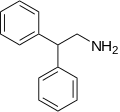 |  |
2,2-Diphenylethylamine made either by reduction of benzhydryl cyanide (Diphenylacetonitrile) or by reaction of Aminoacetaldehyde diethyl acetal with ArH.[15]
Relevant patents
U.S. Patent 6,162,417 U.S. Patent 4,713,386 U.S. Patent 4,719,216 U.S. Patent 4,595,688 U.S. Patent 4,837,328 U.S. Patent 4,572,911
References
- 1 2 Aluisio, L.; Lord, B.; Barbier, A.; Fraser, I.; Wilson, S.; Boggs, J.; Dvorak, L.; Letavic, M.; Maryanoff, B.; Carruthers, N. I.; Bonaventure, P.; Lovenberg, T. W. (2008). "In-vitro and in-vivo characterization of JNJ-7925476, a novel triple monoamine uptake inhibitor". European Journal of Pharmacology. 587 (1–3): 141–146. doi:10.1016/j.ejphar.2008.04.008. PMID 18499098.
- ↑ Maryanoff, B. E.; Mccomsey, D. F.; Castanzo, M. J.; Setler, P. E.; Gardocki, J. F.; Shank, R. P.; Schneider, C. R. (1984). "Pyrroloisoquinoline antidepressants. Potent, enantioselective inhibition of tetrabenazine-induced ptosis and neuronal uptake of norepinephrine, dopamine, and serotonin". Journal of Medicinal Chemistry. 27 (8): 943–946. doi:10.1021/jm00374a001. PMID 6747993.
- ↑ Maryanoff, B. E.; Mccomsey, D. F.; Gardocki, J. F.; Shank, R. P.; Costanzo, M. J.; Nortey, S. O.; Schneider, C. R.; Setler, P. E. (1987). "Pyrroloisoquinoline antidepressants. 2. In-depth exploration of structure-activity relationships". Journal of Medicinal Chemistry. 30 (8): 1433–1454. doi:10.1021/jm00391a028. PMID 3039136.
- ↑ Maryanoff, B. E.; Vaught, J. L.; Shank, R. P.; Mccomsey, D. F.; Costanzo, M. J.; Nortey, S. O. (1990). "Pyrroloisoquinoline antidepressants. 3. A focus on serotonin". Journal of Medicinal Chemistry. 33 (10): 2793–2797. doi:10.1021/jm00172a018. PMID 2213832.
- ↑ Morón, J. A.; Brockington; Wise; Rocha; Hope (2002). "Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines". Journal of Neuroscience. 22 (2): 389–395. PMID 11784783.
- ↑ Maryanoff, B. (1979). "Iminium ion cyclizations. Highly stereoselective synthesis of substituted tetrahydroisoquinoline derivatives". Tetrahedron Letters. 20 (40): 3797–3800. doi:10.1016/S0040-4039(01)95527-3.
- ↑ Maryanoff, B. E.; Mccomsey, D. F.; Duhl-Emswiler, B. A. (1983). "Stereochemistry of intramolecular amidoalkylation reactions in the synthesis of polycyclic isoquinoline derivatives". The Journal of Organic Chemistry. 48 (25): 5062–5074. doi:10.1021/jo00173a053.
- ↑ Maryanoff, B. E.; Mccomsey, D. F.; Almond, H. R.; Mutter, M. S.; Bemis, G. W.; Whittle, R. R.; Olofson, R. A. (1986). "Dramatic reversal of diastereoselectivity in an N-acyliminium ion cyclization leading to hexahydropyrrolo[2,1-a]isoquinolines. A case of competing steric interactions". The Journal of Organic Chemistry. 51 (8): 1341–1346. doi:10.1021/jo00358a034.
- ↑ Maryanoff, B. E.; Mccomsey, D. F.; Mutter, M. S.; Sorgi, K. L.; Maryanuff, C. A. (1988). "Highly stereocontrolled proton transfer in an enammonium-iminium rearrangement. Mechanism of the stereoselective deoxygenation of 6-aryl-6-hydroxy-1,2,3,5,6,10b-hexahydropyrrolo[2.1-]isoquinolines with borane-thf in trifluoroacetic acid". Tetrahedron Letters. 29 (40): 5073–5076. doi:10.1016/S0040-4039(00)80682-6.
- ↑ Mccomsey, D. F.; Maryanoff, B. E. (2000). "3-Aza-cope rearrangement of quaternary N-allyl enammonium salts. Stereospecific 1,3 allyl migration from nitrogen to carbon on a tricyclic template". The Journal of Organic Chemistry. 65 (16): 4938–4943. doi:10.1021/jo000363h. PMID 10956475.
- ↑ Nystrom, Robert F. (1955). "Reduction of Organic Compounds by Mixed Hydrides. I. Nitriles". Journal of the American Chemical Society. 77 (9): 2544–2545. doi:10.1021/ja01614a053.
- ↑ Cha, Jin Soon; Brown, Herbert C. (1993). "Reaction of aluminum hydride-triethylamine complex with selected organic compounds containing representative functional groups". The Journal of Organic Chemistry. 58 (15): 3974–3979. doi:10.1021/jo00067a033.
- ↑ Scully, Frank E. (1980). "Regioselective 2-alkylation and 2-arylation of piperidine and pyrrolidine via organolithiation of cyclic imines". The Journal of Organic Chemistry. 45 (8): 1515–1517. doi:10.1021/jo01296a036.
- ↑ "N-Vinylpyrrolidin-2-One As a 3-Aminopropyl Carbanion Equivalent in the Synthesis of Substituted 1-Pyrrolines: 2-Phenyl-1-Pyrroline". Organic Syntheses. 75: 215. 1998. doi:10.15227/orgsyn.075.0215.
- ↑ Maryanoff, Bruce E.; Nortey, Samuel O.; Gardocki, Joseph F. (1984). "Structure-activity studies on antidepressant 2,2-diarylethylamines". Journal of Medicinal Chemistry. 27 (8): 1067–71. doi:10.1021/jm00374a022. PMID 6747990.