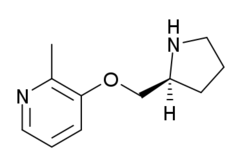
Pozanicline
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 161417-03-4 |
| PubChem (CID) | 178052 |
| ChemSpider | 155000 |
| UNII |
CL2002R563 |
| Chemical and physical data | |
| Formula | C11H16N2O |
| Molar mass | 192.258 |
| 3D model (Jmol) | Interactive image |
| |
| |
| (verify) | |
Pozanicline (ABT-089) is a drug developed by Abbott, that has nootropic and neuroprotective effects.[1][2][3] Animal studies suggested it useful for the treatment of ADHD[4] and subsequent human trials have shown ABT-089 to be effective for this application.[5] It binds with high affinity subtype-selective to the α4β2 nicotinic acetylcholine receptors and has partial agonism to the α6β2 subtype,[6][7] but not the α7 and α3β4 subtypes familiar to nicotine. It has particularly low tendency to cause side effects compared to other drugs in the class[8][9] -making it an exciting candidate for clinical development.
References
- ↑ Lin NH, Gunn DE, Ryther KB, Garvey DS, Donnelly-Roberts DL, Decker MW, Brioni JD, Buckley MJ, Rodrigues AD, Marsh KG, Anderson DJ, Buccafusco JJ, Prendergast MA, Sullivan JP, Williams M, Arneric SP, Holladay MW. Structure-activity studies on 2-methyl-3-(2(S)-pyrrolidinylmethoxy) pyridine (ABT-089): an orally bioavailable 3-pyridyl ether nicotinic acetylcholine receptor ligand with cognition-enhancing properties. Journal of Medicinal Chemistry. 1997 Jan 31;40(3):385-90. PMID 9022806
- ↑ Sullivan JP, Donnelly-Roberts D, Briggs CA, Anderson DJ, Gopalakrishnan M, Xue IC, Piattoni-Kaplan M, Molinari E, Campbell JE, McKenna DG, Gunn DE, Lin NH, Ryther KB, He Y, Holladay MW, Wonnacott S, Williams M, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine]: I. A potent and selective cholinergic channel modulator with neuroprotective properties. Journal of Pharmacology and Experimental Therapeutics. 1997 Oct;283(1):235-46. PMID 9336329
- ↑ Decker MW, Bannon AW, Curzon P, Gunther KL, Brioni JD, Holladay MW, Lin NH, Li Y, Daanen JF, Buccafusco JJ, Prendergast MA, Jackson WJ, Arneric SP. ABT-089 [2-methyl-3-(2-(S)-pyrrolidinylmethoxy)pyridine dihydrochloride]: II. A novel cholinergic channel modulator with effects on cognitive performance in rats and monkeys. Journal of Pharmacology and Experimental Therapeutics. 1997 Oct;283(1):247-58. PMID 9336330
- ↑ Prendergast MA, Jackson WJ, Terry AV Jr, Decker MW, Arneric SP, Buccafusco JJ. Central nicotinic receptor agonists ABT-418, ABT-089, and (−)-nicotine reduce distractibility in adult monkeys. Psychopharmacology (Berlin). 1998 Mar;136(1):50-8. PMID 9537682
- ↑ Wilens TE, Verlinden MH, Adler LA, Wozniak PJ, West SA. ABT-089, a neuronal nicotinic receptor partial agonist, for the treatment of attention-deficit/hyperactivity disorder in adults: results of a pilot study. Biological Psychiatry. 2006 Jun 1;59(11):1065-70. PMID 16499880
- ↑ Marks MJ, Wageman CR, Grady SR, Gopalakrishnan M, Briggs CA (May 2009). "Selectivity of ABT-089 for alpha4beta2* and alpha6beta2* nicotinic acetylcholine receptors in brain". Biochemical Pharmacology. 78 (7): 795–802. doi:10.1016/j.bcp.2009.05.022. PMC 2772152
 . PMID 19481067.
. PMID 19481067. - ↑ Anderson DJ, Malysz J, Grønlien JH, El Kouhen R, Håkerud M, Wetterstrand C, Briggs CA, Gopalakrishnan M (June 2009). "Stimulation of dopamine release by nicotinic acetylcholine receptor ligands in rat brain slices correlates with the profile of high, but not low, sensitivity alpha4beta2 subunit combination". Biochemical Pharmacology. 78 (7): 844–51. doi:10.1016/j.bcp.2009.06.024. PMID 19555668.
- ↑ Rueter LE, Anderson DJ, Briggs CA, Donnelly-Roberts DL, Gintant GA, Gopalakrishnan M, Lin NH, Osinski MA, Reinhart GA, Buckley MJ, Martin RL, McDermott JS, Preusser LC, Seifert TR, Su Z, Cox BF, Decker MW, Sullivan JP. ABT-089: pharmacological properties of a neuronal nicotinic acetylcholine receptor agonist for the potential treatment of cognitive disorders. CNS Drug Reviews. 2004 Summer;10(2):167-82. PMID 15179445
- ↑ Wilens TE, Decker MW. Neuronal nicotinic receptor agonists for the treatment of attention-deficit/hyperactivity disorder: focus on cognition. Biochemical Pharmacology. 2007 Oct 15;74(8):1212-23. doi:10.1016/j.bcp.2007.07.002 PMID 17689498
This article is issued from Wikipedia - version of the 6/5/2016. The text is available under the Creative Commons Attribution/Share Alike but additional terms may apply for the media files.