Neutrophil extracellular traps
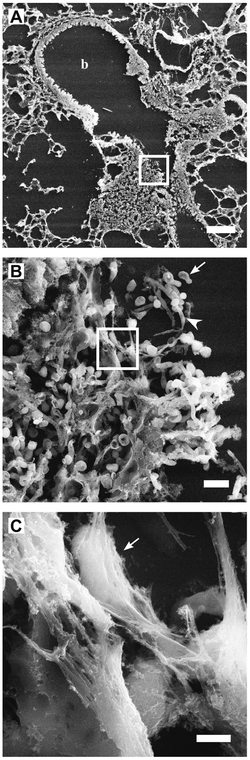
Neutrophil extracellular traps (NETs) are networks of extracellular fibers, primarily composed of DNA from neutrophils, which bind pathogens.[1]
It has long been known that neutrophils (the immune system's front-line of defense against infection) use two strategies to kill invading pathogens: engulfment of microbes and secretion of anti-microbials. In 2004, a novel third function was identified: formation of NETs, whereby neutrophils kill extracellular pathogens while minimizing damage to the host cells. Upon in vitro activation with the pharmacological agent phorbol myristate acetate (PMA), Interleukin 8 (IL-8) or lipopolysaccharide (LPS), neutrophils release granule proteins and chromatin to form an extracellular fibril matrix known as NETs through an active process.[1]
NETs disarm pathogens with antimicrobial proteins such as neutrophil elastase, cathepsin G and histones that have a high affinity for DNA.[2] Analysis by immunofluorescence corroborated that NETs contained proteins from azurophilic granules (neutrophil elastase, cathepsin G and myeloperoxidase) as well as proteins from specific granules (lactoferrin) and tertiary granules (gelatinase) and cytoplasm, yet CD63, actin, tubulin and various other cytoplasmatic proteins were not.[1][3] NETs provide for a high local concentration of antimicrobial components and bind, disarm, and kill microbes extracellularly independent of phagocytic uptake. In addition to their antimicrobial properties, NETs may serve as a physical barrier that prevents further spread of the pathogens. Furthermore, delivering the granule proteins into NETs may keep potentially injurious proteins like proteases from diffusing away and inducing damage in tissue adjacent to the site of inflammation.
High-resolution scanning electron microscopy has shown that NETs consist of stretches of DNA and globular protein domains with diameters of 15-17 nm and 25 nm, respectively. These aggregate into larger threads with a diameter of 50 nm.[1] However, under flow conditions, NETs can form much larger structures, hundreds of nanometers in length and width.[4]
More recently, it has also been shown that not only bacteria but also pathogenic fungi such as Candida albicans induces neutrophils to form NETs that capture and kill C. albicans hyphal as well as yeast-form cells.[5] NETs have also been documented in association with Plasmodium falciparum infections in children.[6] NETs might also have a deleterious effect on the host, because the exposure of extracellular histone complexes could play a role during the development of autoimmune diseases like systemic lupus erythematosus.[7] NETs could also play a role in inflammatory diseases, as NETs could be identified in preeclampsia, a pregnancy related inflammatory disorder in which neutrophils are known to be activated.[8] NETs have also been reported in the colon mucosa of patients with the inflammatory bowel disease ulcerative colitis.[9] NETs have also been associated with the production of IgG antinuclear double stranded DNA antibodies in children infected with P. falciparum malaria.[6]
While it was originally proposed that NETs would be formed in tissues at a site of bacterial/yeast infection, NETs have also been shown to form within blood vessels during sepsis (specifically in the lung capillaries and liver sinusoids). Intra-vascular NET formation is tightly controlled and is regulated by platelets, which sense severe infection via platelet TLR4 and then bind to and activate neutrophils to form NETs. Platelet-induced NET formation occurs very rapidly (in minutes) and does not result in death of the neutrophils. NETs formed in blood vessels can catch circulating bacteria as they pass through the vessels. Trapping of bacteria under flow has been imaged directly in flow chambers in vitro and intravital microscopy demonstrated that bacterial trapping occurs in the liver sinusoids and lung capillaries (sites where platelets bind neutrophils).[4] NETs also have a role in thrombosis.
The formation of NETs is regulated by the lipoxygenase pathway – during certain forms of activation (including contact with bacteria) neutrophil 5-lipoxygenase forms 5-HETE-phospholipids that inhibit NET formation.[10] Evidence from laboratory experiments suggests that NETs are cleaned away by macrophages that take them to themselves and degrade them.[11]
These observations suggest that NETs might play an important role in the pathogenesis of infectious, inflammatory and thrombotic disorders.[12][13][14]
References
- 1 2 3 4 Brinkmann, Volker; Ulrike Reichard; Christian Goosmann; Beatrix Fauler; Yvonne Uhlemann; David S. Weiss; Yvette Weinrauch; Arturo Zychlinsky (2004-03-05). "Neutrophil Extracellular Traps Kill Bacteria". Science. AAAS. 303 (5663): 1532–1535. doi:10.1126/science.1092385. PMID 15001782. Retrieved 2007-04-09.
- ↑ Thomas MP, Whangbo J, McCrossan G, et al. (June 2014). "Leukocyte protease binding to nucleic acids promotes nuclear localization and cleavage of nucleic acid binding proteins". Journal of Immunology. 192 (11): 5390–7. doi:10.4049/jimmunol.1303296. PMC 4041364
 . PMID 24771851.
. PMID 24771851. - ↑ Urban CF, Ermert D, Schmid M, Abu-Abed U, Goosmann C, Nacken W, Brinkmann V, Jungblut PR, Zychlinsky A (2009). "Neutrophil extracellular traps contain calprotectin, a cytosolic protein complex involved in host defense against Candida albicans.". PLOS Pathogens. 5: e1000639. doi:10.1371/journal.ppat.1000639. PMC 2763347
 . PMID 19876394.
. PMID 19876394. - 1 2 Clark SR, Ma AC, Tavener SA, McDonald B, Goodarzi Z, Kelly MM, Patel KD, Chakrabarti S, McAvoy E, Sinclair GD, Keys EM, Allen-Vercoe E, Devinney R, Doig CJ, Green FH, Kubes P (2007). "Platelet Toll-Like Receptor-4 Activates Neutrophil Extracellular Traps to Ensnare Bacteria in Endotoxemic and Septic Blood" (PDF). Nature Medicine. 13 (4): 463–9. doi:10.1038/nm1565. PMID 17384648.
- ↑ Urban, CF; Reichard U; Brinkmann V; Zychlinsky A (April 2006). "Neutrophil extracellular traps capture and kill Candida albicans yeast and hyphal forms". Cell Microbiol. 8 (4): 668–76. doi:10.1111/j.1462-5822.2005.00659.x. PMID 16548892.
- 1 2 Baker VS, Imade GE, Molta NB, Tawde P, Pam SD, Obadofin MO, Sagay SA, Egah DZ, Iya D, Afolabi BB, Baker M, Ford K, Ford R, Roux KH, Keller TC (February 2008). "Cytokine-associated neutrophil extracellular traps and antinuclear antibodies in Plasmodium falciparum infected children under six years of age". Malaria Journal. 7 (41): 41. doi:10.1186/1475-2875-7-41. PMC 2275287
 . PMID 18312656.
. PMID 18312656. - ↑ Hakkim A, Fürnrohr BG, Amann K, Laube B, Abed UA, Brinkmann V, Herrmann M, Voll RE, Zychlinsky A (2010). "Impairment of neutrophil extracellular trap degradation is associated with lupus nephritis". Proc Natl Acad Sci U S A. 107 (21): 9813–8. doi:10.1073/pnas.0909927107. PMC 2906830
 . PMID 20439745.
. PMID 20439745. - ↑ Gupta, AK; Hasler P; Holzgreve W; Gebhardt S; Hahn S. (November 2005). "Induction of neutrophil extracellular DNA lattices by placental microparticles and IL-8 and their presence in preeclampsia". Hum Immunol. 66 (11): 1146–54. doi:10.1016/j.humimm.2005.11.003. PMID 16571415.
- ↑ Bennike, Tue Bjerg; Carlsen, Thomas Gelsing; Ellingsen, Torkell; Bonderup, Ole Kristian; Glerup, Henning; Bøgsted, Martin; Christiansen, Gunna; Birkelund, Svend; Stensballe, Allan. "Neutrophil Extracellular Traps in Ulcerative Colitis". Inflammatory Bowel Diseases. 21 (9): 2052–2067. doi:10.1097/mib.0000000000000460.
- ↑ Clark, SR; Guy CJ; Scurr MJ; Taylor PR; Kift-Morgan AP; Hammond VJ; Thomas CP; Coles B; Roberts GW; Eberl M; Jones SA; Topley N; Kotecha S; O'Donnell VB (2011). "Esterified eicosanoids are acutely generated by 5-lipoxygenase in primary human neutrophils and in human and murine infection". Blood. 117 (6): 2033–43. doi:10.1182/blood-2010-04-278887. PMC 3374621
 . PMID 21177434.
. PMID 21177434. - ↑ Farrera, c; Fadeel B (2013). "Macrophage Clearance of Neutrophil Extracellular Traps Is a Silent Process". Journal of Immunology. 191 (5): 2647–56. doi:10.4049/jimmunol.1300436. PMID 23904163.
- ↑ Fuchs TA, Brill A, Duerschmied D, Schatzberg D, Monestier M, Myers DD, Wrobleski SK, Wakefield TW, Hartwig JH, Wagner DD (Sep 7, 2010). "Extracellular DNA traps promote thrombosis". Proc Natl Acad Sci U S A. 107 (36): 15880–5. doi:10.1073/pnas.1005743107. PMC 2936604
 . PMID 20798043.
. PMID 20798043. - ↑ Brill A, Fuchs TA, Savchenko AS, Thomas GM, Martinod K, De Meyer SF, Bhandari AA, Wagner DD (Nov 1, 2011). "Neutrophil Extracellular Traps Promote Deep Vein Thrombosis in Mice". Journal of thrombosis and haemostasis : JTH. 10: 136–144. doi:10.1111/j.1538-7836.2011.04544.x. PMC 3319651
 . PMID 22044575.
. PMID 22044575. - ↑ Borissoff, JI; ten Cate, H (September 2011). "From neutrophil extracellular traps release to thrombosis: an overshooting host-defense mechanism?". Journal of thrombosis and haemostasis : JTH. 9 (9): 1791–4. doi:10.1111/j.1538-7836.2011.04425.x. PMID 21718435.
External links
- Frontiers in Molecular Innate Immunity research topic about NET
- "Molecular mechanisms involved in neutrophil extracellular trap (NET) formation", PhD thesis, Jyaysi Desai, Ludwig Maximilian University of Munich, Germany. Chemistry winner, Dance Your PhD, 2015.