Stishovite
| Stishovite | |
|---|---|
|
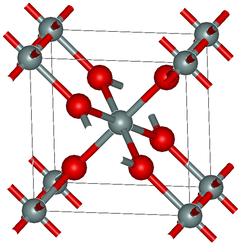
Crystal structure of stishovite | |
| General | |
| Category | Tectosilicate |
| Formula (repeating unit) | SiO2 |
| Strunz classification | 4.DA.40 (Oxides) |
| Crystal system | Tetragonal |
| Crystal class |
Ditetragonal dipyramidal (4/mmm) H–M symbol: (4/m 2/m 2>/m) |
| Space group | P42/mnm (No. 136) |
| Unit cell |
a = 4.1772(7), c = 2.6651(4) [Å]; Z = 2 |
| Identification | |
| Color | Colorless (when pure) |
| Mohs scale hardness | 9–9.5[1] |
| Luster | Vitreous |
| Diaphaneity | Transparent to translucent |
| Specific gravity | 4.287 |
| Optical properties | Uniaxial (+) |
| Refractive index | nω = 1.799–1.800 nε = 1.826–1.845 |
| Birefringence | δ = 0.027 |
| Melting point | (decomposes) |
| References | [2][3][4] |
Stishovite is an very hard, dense tetragonal form (polymorph) of silicon dioxide. It is very rare on the Earth's surface, however, it may be a predominant form of silicon dioxide in the Earth, especially in the mantle.[5]
Stishovite was named after Sergey M. Stishov, a Russian high-pressure physicist who first synthesized the mineral in 1961. It was discovered in Meteor Crater in 1962 by Edward C. T. Chao.[6]
Unlike other silica polymorphs, the crystal structure of stishovite resembles that of rutile (TiO2). The silicon in stishovite adopts an octahedral coordination geometry, being bound to six oxides. Similarly, the oxides are three-connected, unlike low-pressure forms of SiO2. In most silicates, silicon is tetrahedral, being bound to four oxides.[7] It was long considered the hardest known oxide (~30 GPa Vickers[1]); however, boron suboxide has been discovered[8] in 2002 to be much harder. At normal temperature and pressure, stishovite is metastable.
Stishovite can be separated from quartz by applying hydrogen fluoride (HF); unlike quartz, stishovite will not react.[6]
Appearance
Large natural crystals of stishovite are extremely rare and are usually found as clasts of 1 to 2 mm in length. When found, they can be difficult to distinguish from regular quartz without laboratory analysis. It has a vitreous luster, is transparent (or translucent), and is extremely hard. Stishovite usually sits as small rounded gravels in a matrix of other minerals.
Synthesis
Until recently, the only known occurrences of stishovite in nature formed at the very high shock pressures (>100 kbar or 10 GPa) and temperatures (> 1200 °C) present during hypervelocity meteorite impact into quartz-bearing rock. Minute amounts of stishovite have been found within diamonds,[9] and post-stishovite phases were identified within ultra-high-pressure mantle rocks.[10] Stishovite may also be synthesized by duplicating these conditions in the laboratory, either isostatically or through shock (see shocked quartz).[11] At 4.287 g/cm3 it is second densest polymorph of silica, after seifertite. It has tetragonal crystal symmetry, P42/mnm, No.136, Pearson symbol tP6.[12]
See also
- Coesite, a related mineral
References
- 1 2 Luo, Sheng-Nian; Swadener, J.G.; Ma, Chi; Tschauner, Oliver (2007). "Examining crystallographic orientation dependence of hardness of silica stishovite" (PDF). Physica B: Condensed Matter. 399 (2): 138. Bibcode:2007PhyB..399..138L. doi:10.1016/j.physb.2007.06.011. and references therein
- ↑ Anthony, John W.; Bideaux, Richard A.; Bladh, Kenneth W.; Nichols, Monte C., eds. (1995). "Stishovite". Handbook of Mineralogy (PDF). II (Silica, Silicates). Chantilly, VA, US: Mineralogical Society of America. ISBN 0962209716. Retrieved December 5, 2011.
- ↑ Stishovite. Mindat.org
- ↑ Stishovite. Webmineral.com
- ↑ Dmitry L. Lakshtanov et al. "The post-stishovite phase transition in hydrous alumina-bearing SiO2 in the lower mantle of the earth" PNAS 2007 104 (34) 13588-13590; doi:10.1073/pnas.0706113104
- 1 2 Fleischer, Michael (1962). "New mineral names" (PDF). American Mineralogist. Mineralogical Society of America. 47 (2): 172–174.
- ↑ Ross, Nancy L. (1990). "High pressure crystal chemistry of stishovite" (PDF). American Mineralogist. Mineralogical Society of America. 75 (7): 739–747.
- ↑ He, Duanwei; Zhao, Yusheng; Daemen, L.; Qian, J.; Shen, T. D.; Zerda, T. W. (2002). "Boron suboxide: As hard as cubic boron nitride". Applied Physics Letters. 81 (4): 643. doi:10.1063/1.1494860.
- ↑ Wirth, R; Vollmer, C; Brenker, F; Matsyuk, S; Kaminsky, F (2007). "Inclusions of nanocrystalline hydrous aluminium silicate "Phase Egg" in superdeep diamonds from Juina (Mato Grosso State, Brazil)". Earth and Planetary Science Letters. 259 (3–4): 384. Bibcode:2007E&PSL.259..384W. doi:10.1016/j.epsl.2007.04.041.
- ↑ Liu, L; Zhang, J; Greenii, H; Jin, Z; Bozhilov, K (2007). "Evidence of former stishovite in metamorphosed sediments, implying subduction to >350 km" (PDF). Earth and Planetary Science Letters. 263 (3–4): 180. Bibcode:2007E&PSL.263..180L. doi:10.1016/j.epsl.2007.08.010.
- ↑ J. M. Léger, J. Haines, M. Schmidt, J. P. Petitet, A. S. Pereira & J. A. H. da Jornada (1996). "Discovery of hardest known oxide". Nature. 383 (6599): 401. Bibcode:1996Natur.383..401L. doi:10.1038/383401a0.
- ↑ Smyth J.R.; Swope R.J.; Pawley A.R. (1995). "H in rutile-type compounds: II. Crystal chemistry of Al substitution in H-bearing stishovite" (PDF). American Mineralogist. 80: 454–456.