Sulfoxide
A sulfoxide is a chemical compound containing a sulfinyl (SO) functional group attached to two carbon atoms. It is a polar functional group. Sulfoxides are the oxidized derivatives of sulfides. Examples of important sulfoxides are alliin, a precursor to the compound that gives freshly crushed garlic its aroma, and DMSO, a common solvent.[1]
Structure and bonding
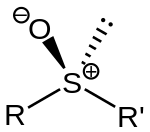
Sulfoxides feature a pyramidal sulfur center with relatively short S-O distances. In DMSO, the S-O distance is 1.531 Å.[2]
Sulfoxides are generally represented with the structural formula R–S(=O)–R', where R and R' are organic groups. The bond between the sulfur and oxygen atoms is intermediate of a dative bond and a polarized double bond.[3] The S–O interaction has an electrostatic aspect, resulting in significant dipolar character, with negative charge centered on oxygen. A lone pair of electrons resides on the sulfur atom giving it tetrahedral electron pair geometry and trigonal pyramidal shape (steric number 4 with one lone pair; see VSEPR theory). When the two organic residues are dissimilar, the sulfur is a chiral center, for example, methylphenylsulfoxide.
The energy required to invert this stereocenter is sufficiently high that sulfoxides are optically stable, that is, the rate of racemization is slow at room temperature.
Preparation
Sulfoxides are typically prepared by oxidation of sulfides.[4] A typical oxidant is hydrogen peroxide. Oxidation of thioanisole can be effected with periodate.[5] In these oxidations, care is required to avoid over oxidation to the sulfone. Dimethyl sulfide is oxidized to dimethyl sulfoxide and then to dimethyl sulfone.[6] Unsymmetrical sulfides are prochiral, thus their oxidation gives chiral sulfoxides. Certain reagents or catalysts effect enantioselective oxidations.
Diaryl sulfoxides can be prepared by Friedel-Crafts arylation of sulfur dioxide using acid catalyst:
- 2 ArH + SO2 → Ar2SO + H2O
Reactions
Sulfoxides, such as DMSO, have basic character, being excellent ligands and readily alkylated. Similarly, they can be oxidized to sulfones.
Alkyl sulfoxides are susceptible to deprotonation of α-CH groups by strong bases, such as sodium hydride:[7]
- CH3S(O)CH3 + NaH → CH3S(O)CH2Na + H2
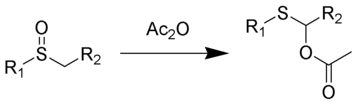
In the Pummerer rearrangement, alkyl sulfoxides react with acetic anhydride to give migration of the oxygen from sulfur to the adjacent carbon as an acetate ester.
Sulfoxides form complexes with transition metals.[8]
-ruthenium(II)-3D-balls.png) RuCl2(dmso)4, a representative metal complex of a sulfoxide. The DMSO is coordinated to Ru in two ways, through O and through S.
RuCl2(dmso)4, a representative metal complex of a sulfoxide. The DMSO is coordinated to Ru in two ways, through O and through S.
Applications
Chiral sulfoxides find application in certain drugs such as esomeprazole and armodafinil, and they are also employed as chiral auxiliaries.[9] DMSO is widely used as a solvent in the laboratory.
Natural occurrence
Methionine sulfoxide forms from the amino acid methionine and its accumulation is associated with aging. The enzyme DMSO reductase catalyzes the interconversion of DMSO and dimethylsulfide.
References
- ↑ "Syntheses of Sulphones, Sulphoxides and Cyclic Sulphides" Saul Patai, Zvi Rappoport, Eds., 1995, John Wiley & Sons. ISBN 9780470666357. doi:10.1002/9780470666357
- ↑ R. Thomas, C. B. Shoemaker and K. Eriks "The molecular and crystal structure of dimethyl sulfoxide, (H3C)2SO Acta Crystallogr. 1966, vol. 21, 12-20. doi:10.1107/S0365110X66002263,
- ↑ Terence P. Cunningham, David L. Cooper, Joseph Gerratt, Peter B. Karadakov and Mario Raimondi (1997). "Chemical bonding in oxofluorides of hypercoordinate sulfur". Journal of the Chemical Society, Faraday Transactions. 93 (13): 2247–2254. doi:10.1039/A700708F.
- ↑ Kathrin-Maria Roy "Sulfones and Sulfoxides" Ullmann's Encyclopedia of Industrial Chemistry 2002, Wiley-VCH, Weinheim. doi:10.1002/14356007.a25_487
- ↑ Carl R. Johnson, Jeffrey E. Keiser "Methyl Phenyl Sulfoxide" Org. Synth. 1966, volume 46, 78. doi:10.15227/orgsyn.046.0078
- ↑ http://www.chem.harvard.edu/groups/myers/handouts/10_Directed_Ortho.pdf
- ↑ Iwai, I.; Ide, J. (1988). "2,3-Diphenyl-1,3-Butadiene". Org. Synth.; Coll. Vol., 6, p. 531
- ↑ Mario Calligaris "Structure and bonding in metal sulfoxide complexes: an update" Coordination Chemistry Reviews 2004, vol. 248, pp. 351-375. doi:10.1016/j.ccr.2004.02.005
- ↑ Oxidation of sulfides to chiral sulfoxides using Schiff base-vanadium (IV) complexes Ángeles Gama, Lucía Z. Flores-López, Gerardo Aguirre, Miguel Parra-Hake, Lars H. Hellberg, and Ratnasamy Somanathan Arkivoc MX-789E 2003 Online article