Oxime

An oxime is a chemical compound belonging to the imines, with the general formula R1R2C=N O H, where R1 is an organic side-chain and R2 may be hydrogen, forming an aldoxime, or another organic group, forming a ketoxime. O-substituted oximes form a closely related family of compounds. Amidoximes are oximes of amides with general structure RC(=NOH)(NRR').
Oximes are usually generated by the reaction of hydroxylamine and aldehydes or ketones. The term oxime dates back to the 19th century, a combination of the words oxygen and imine.[1]
Structure and properties
If the two side-chains on the central carbon are different from each other, the oxime can have two geometric stereoisomeric form: a syn isomer and an anti isomer, depending on which of the two side-chains is closer to the hydroxyl. Both forms are often stable enough to be separated from each other by standard techniques.
Oximes have three characteristic bands in the infrared spectrum, at wavenumbers 3600 (O-H), 1665 (C=N) and 945 (N-O).[2]
In aqueous solution, aliphatic oximes are 102- to 103-fold more resistant to hydrolysis than analogous hydrazones.[3]
Preparation
Oximes can be synthesized by condensation of an aldehyde or a ketone with hydroxylamine. The condensation of aldehydes with hydroxylamine gives aldoxime, and ketoxime is produced from ketones and hydroxylamine. In general, oximes exist as colorless crystals and are poorly soluble in water. Therefore, oximes can be used for the identification of ketone or aldehyde.
Oximes can also be obtained from reaction of nitrites such as isoamyl nitrite with compounds containing an acidic hydrogen atom. Examples are the reaction of ethyl acetoacetate and sodium nitrite in acetic acid,[4][5] the reaction of methyl ethyl ketone with ethyl nitrite in hydrochloric acid.[6] and a similar reaction with propiophenone,[7] the reaction of phenacyl chloride,[8] the reaction of malononitrile with sodium nitrite in acetic acid[9]
A conceptually related reaction is the Japp–Klingemann reaction.
Reactions
The hydrolysis of oximes proceeds easily by heating in the presence of various inorganic acids, and the oximes decompose into the corresponding ketones or aldehydes, and hydroxylamines. The reduction of oximes by sodium metal,[10] sodium amalgam, hydrogenation, or reaction with hydride reagents produces amines.[11] Typically the reduction of aldoximes gives both primary amines and secondary amines; however, reaction conditions can be altered (such as the addition of potassium hydroxide in a 1/30 molar ratio) to yield solely primary amines.[12]
In general, oximes can be changed to the corresponding amide derivatives by treatment with various acids. This reaction is called Beckmann rearrangement. In this reaction, a hydroxyl group is exchanged with the group that is in the anti position of the hydroxyl group. The amide derivatives that are obtained by Beckmann rearrangement can be transformed into a carboxylic acid by means of hydrolysis (base or acid catalyzed). And an amine by hoffman degradation of the amide in the presence of alkali hypoclorites at 80 degrees Celsius, the degradation is itself prone to side reactions, namely the formation of biurets or cyanate polymers., To avoid this side-reaction, strict temperature control is necessary; the reaction must be conducted at sufficient temperature to isomerise the cyanate to the isocyante. Also, good solvation is also crucial to be successful. Beckmann rearrangement is used for the industrial synthesis of caprolactam (see applications below).
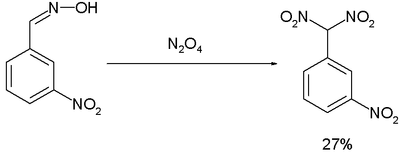
The Ponzio reaction (1906)[13] concerning the conversion of m-nitrobenzaldoxime to m-nitrophenyldinitromethane with dinitrogen tetroxide was the result of research into TNT-like high explosives:[14]
In the Neber rearrangement certain oximes are converted to the corresponding alpha-amino ketones.
Oximes can be dehydrated using acid anhydrides to yield corresponding nitriles.
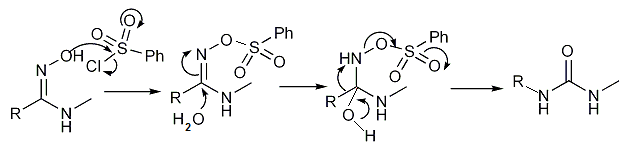
Certain amidoximes react with benzenesulfonyl chloride to substituted ureas in the Tiemann rearrangement[15][16]
Uses
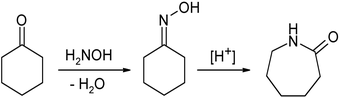
In their largest application, an oxime is an intermediate in the industrial production of caprolactam, a precursor to Nylon 6. About half of the world's supply of cyclohexanone, more than a billion kilograms annually, is converted to the oxime. In the presence of sulfuric acid catalyst, the oxime undergoes the Beckmann rearrangement to give the cyclic amide caprolactam:[17]
Metal extractants
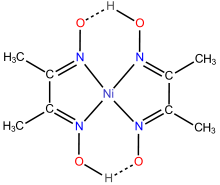
Oximes are commonly used as ligands and sequestering agents for metal ions. Dimethylglyoxime (dmgH2) is a reagent for the analysis of nickel and a popular ligand in its own right. In the typical reaction, a metal reacts with two equivalents of dmgH2 concomitant with ionization of one proton. Salicylaldoxime is a chelator and an extractant in hydrometallurgy.[18]
Some amidoximes like polyacrylamidoxime can be used to capture trace amounts of uranium from sea water[19][20]
Other applications
- Oxime compounds are used as antidotes for nerve agents. A nerve agent inactivates acetylcholinesterase molecules by phosphorylation of the molecule. Oxime compounds can reactivate acetylcholinesterase by attaching to the phosphorus atom and forming an oxime-phosphonate, which then splits away from the acetylcholinesterase molecule. The most effective oxime nerve-agent antidotes are pralidoxime (also known as 2-PAM), obidoxime, methoxime, HI-6, Hlo-7, and TMB-4.[21] The effectiveness of the oxime treatment depends on the particular nerve agent used.[22]
- Perillartine, the oxime of perillaldehyde is used as an artificial sweetener in Japan, as it is 2000 times sweeter than sucrose.
- Diaminoglyoxime, a glyoxime derivative, is a key precursor to various compounds, containing the highly reactive furazan ring.
- Methyl ethyl ketoxime is a skin-preventing additive in many oil-based paints.
See also
References
- ↑ The name "oxime" is derived from "oximide" (i.e., oxy- + amide). According to the German organic chemist Victor Meyer (1848–1897) – who, with Alois Janny, synthesized the first oximes – an "oximide" was an organic compound containing the group (=N-OH) attached to a carbon atom. The existence of oximides was questioned at the time (ca. 1882). (See page 1164 of: Victor Meyer und Alois Janny (1882a) "Ueber stickstoffhaltige Acetonderivate" (On nitrogenous derivatives of acetone), Berichte der Deutschen chemischen Gesellschaft, 15: 1164–1167.) However, in 1882, Meyer and Janny succeeded in synthesizing methylglyoxime (CH3C(=NOH)CH(=NOH)), which they named "Acetoximsäure" (acetoximic acid) (Meyer & Janny, 1882a, p. 1166). Subsequently, they synthesized 2-propanone, oxime ((CH3)2C=NOH), which they named "Acetoxim" (acetoxime), in analogy with Acetoximsäure. From Victor Meyer and Alois Janny (1882b) "Ueber die Einwirkung von Hydroxylamin auf Aceton" (On the effect of hydroxylamine on acetone), Berichte der Deutschen chemischen Gesellschaft, 15: 1324–1326, page 1324: "Die Substanz, welche wir, wegen ihrer nahen Beziehungen zur Acetoximsäure, und da sie keine sauren Eigenschaften besitzt, vorläufig Acetoxim nennen wollen, …" (The substance, which we – on account of its close relations to acetoximic acid, and since it possesses no acid properties – will, for the present, name "acetoxime," … )
- ↑ Reusch, W. "Infrared Spectroscopy". Virtual Textbook of Organic Chemistry. Michigan State University.
- ↑ Kalia, J.; Raines, R. T. (2008). "Hydrolytic stability of hydrazones and oximes". Angew. Chem. Int. Ed. 47 (39): 7523–7526. doi:10.1002/anie.200802651. PMC 2743602
 . PMID 18712739.
. PMID 18712739. - ↑ Fischer, Hans (1943). "2,4-Dimethyl-3,5-dicarbethoxypyrrole". Org. Synth.; Coll. Vol., 2, p. 202
- ↑ Fischer, Hans (1955). "Kryptopyrrole". Org. Synth.; Coll. Vol., 3, p. 513
- ↑ Semon, W. L. and Damerell, V. R. (1943). "Dimethoxyglyoxime". Org. Synth.; Coll. Vol., 2, p. 204
- ↑ Hartung, Walter H. and Crossley, Frank (1943). "Isonitrosopropiophenone". Org. Synth.; Coll. Vol., 2, p. 363
- ↑ Levin, Nathan and Hartung, Walter H. (1955). "ω-chloroisonitrosoacetophenone". Org. Synth.; Coll. Vol., 3, p. 191
- ↑ Ferris, J. P.; Sanchez, R. A. and Mancuso, R. W. (1973). "p-toluenesulfonate". Org. Synth.; Coll. Vol., 5, p. 32
- ↑ Suter, C. M.; Moffett, Eugene W. (1934). "The Reduction of Aliphatic Cyanides and Oximes with Sodium and n-Butyl Alcohol". Journal of the American Chemical Society. 56 (2): 487–487. doi:10.1021/ja01317a502.
- ↑ George, Frederick; Saunders, Bernard (1960). Practical Organic Chemistry, 4th Ed. London: Longman. p. 93 & 226. ISBN 9780582444072.
- ↑ Hata, Kazuo (1972). New Hydrogenating Catalysts. New York: John Wiley & Sons Inc. p. 193. ISBN 9780470358900.
- ↑ Ponzio, Giacomo (1906). "Einwirkung von Stickstofftetroxyd auf Benzaldoxim". J. Prakt. Chem. 73: 494. doi:10.1002/prac.19060730133.
- ↑ Fieser, Louis F. and Doering, William von E. (1946). "Aromatic-Aliphatic Nitro Compounds. III. The Ponzio Reaction; 2,4,6-Trinitrobenzyl Nitrate". J. Am. Chem. Soc. 68 (11): 2252. doi:10.1021/ja01215a040.
- ↑ Tiemann, Ferdinand (1891). "Ueber die Einwirkung von Benzolsulfonsäurechlorid auf Amidoxime". Chemische Berichte. 24 (2): 4162–4167. doi:10.1002/cber.189102402316.
- ↑ Plapinger, Robert; Owens, Omer (1956). "Notes – The Reaction of Phosphorus-Containing Enzyme Inhibitors with Some Hydroxylamine Derivatives". J. Org. Chem. 21 (10): 1186. doi:10.1021/jo01116a610.
- ↑ Ritz, Josef; Fuchs, Hugo; Kieczka, Heinz; Moran, William C. (2005), "Caprolactam", Ullmann's Encyclopedia of Industrial Chemistry, Weinheim: Wiley-VCH, doi:10.1002/14356007.a05_031.pub2
- ↑ Smith, Andrew G.; Tasker, Peter A.; White, David J. "The structures of phenolic oximes and their complexes" Coordination Chemistry Reviews 2003, volume24, pp. 61–85. doi:10.1016/S0010-8545(02)00310-7
- ↑ Rao, Linfeng. "Recent International R&D Activities in the Extraction of Uranium from Seawater". Lawrence Berkeley National Laboratory. Retrieved 21 September 2012.
- ↑ Kanno, M (1984). "Present status of study on extraction of uranium from sea water". Journal of Nuclear Science and Technology. Journal of Nuclear Science and Technology. 21: 1. doi:10.1080/18811248.1984.9731004. Retrieved 21 September 2012.
- ↑ Rowe, Aaron (27 November 2007). "New Nerve Gas Antidotes". Wired (magazine).
- ↑ Kassa, J. (2002). "Review of oximes in the antidotal treatment of poisoning by organophosphorus nerve agents". Journal of Toxicology – Clinical Toxicology. 40 (6): 803–16. doi:10.1081/CLT-120015840. PMID 12475193.