Leucine
 L-Leucine | |
 L-Leucine at physiological pH | |
| Names | |
|---|---|
| IUPAC name
Leucine | |
| Other names
2-Amino-4-methylpentanoic acid | |
| Identifiers | |
| 61-90-5 | |
| 3D model (Jmol) | Interactive image |
| ChEBI | CHEBI:57427 |
| ChEMBL | ChEMBL291962 |
| ChemSpider | 5880 |
| DrugBank | DB01746 |
| ECHA InfoCard | 100.000.475 |
| 3312 | |
| KEGG | D00030 |
| PubChem | 6106 |
| UNII | GMW67QNF9C |
| |
| |
| Properties | |
| C6H13NO2 | |
| Molar mass | 131.18 g·mol−1 |
| Acidity (pKa) | 2.36 (carboxyl), 9.60 (amino)[1] |
| Supplementary data page | |
| Refractive index (n), Dielectric constant (εr), etc. | |
| Thermodynamic data |
Phase behaviour solid–liquid–gas |
| UV, IR, NMR, MS | |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Leucine (abbreviated as Leu or L; encoded by the six codons UUA, UUG, CUU, CUC, CUA, and CUG) is an α-amino acid used in the biosynthesis of proteins. It contains an α-amino group (which is in the protonated −NH+
3 form under biological conditions), an α-carboxylic acid group (which is in the deprotonated −COO− form under biological conditions), and an isobutyl side chain, classifying it as a nonpolar (at physiological pH) amino acid. It is essential in humans—meaning the body cannot synthesize it and thus must obtain from the diet.
Leucine is a major component of the subunits in ferritin, astacin, and other "buffer" proteins.
Biology
Leucine is used in the liver, adipose tissue, and muscle tissue. Adipose and muscle tissue use leucine in the formation of sterols. Combined leucine use in these two tissues is seven times greater than in the liver.[2]
Biosynthesis in plants
Although it is an essential amino acid, animals cannot synthesize leucine. Consequently, they must ingest it, usually as a component of proteins. Plants and microorganisms synthesize leucine from pyruvic acid with a series of enzymes:[3]
- Acetolactate synthase
- Acetohydroxy acid isomeroreductase
- Dihydroxyacid dehydratase
- α-Isopropylmalate synthase
- α-Isopropylmalate isomerase
- Leucine aminotransferase
Synthesis of the small, hydrophobic amino acid valine also includes the initial part of this pathway.
Metabolism in humans
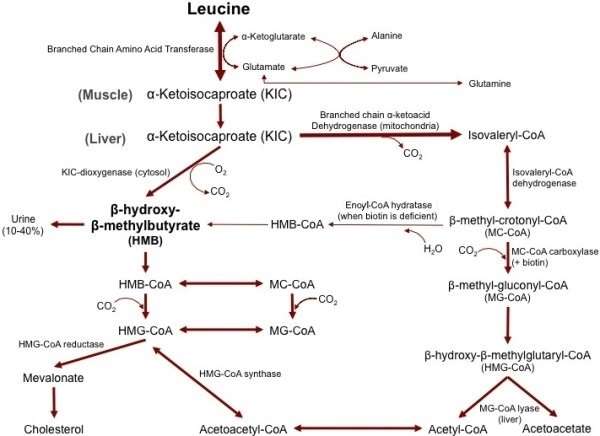
In healthy individuals, approximately 60% of dietary L-leucine is metabolized after several hours, with roughly 5% (2–10% range) of dietary L-leucine being converted to β-hydroxy β-methylbutyric acid (HMB).[5][6][7] Around 40% of dietary L-leucine is converted to acetyl-CoA, which is subsequently used in the synthesis of other compounds.[5]
The vast majority of L-leucine metabolism is initially catalyzed by the branched-chain amino acid aminotransferase enzyme, producing α-ketoisocaproate (α-KIC).[5][6] α-Ketoisocaproate is mostly metabolized by the mitochondrial enzyme branched-chain α-ketoacid dehydrogenase, which converts it to isovaleryl-CoA.[5][6] Isovaleryl-CoA is subsequently metabolized by isovaleryl-CoA dehydrogenase and converted to β-methylcrotonoyl-CoA (MC-CoA), which is used in the synthesis of acetyl-CoA and other compounds.[5] During biotin deficiency, HMB can be synthesized from MC-CoA via enoyl-CoA hydratase and an unknown thioesterase enzyme,[8][9][10] which convert MC-CoA into β-hydroxy β-methylbutyryl-CoA (HMB-CoA) and HMB-CoA into HMB respectively.[10] A relatively small amount of α-KIC is metabolized in the liver by the cytosolic enzyme 4-hydroxyphenylpyruvate dioxygenase (KIC dioxygenase), which converts α-KIC to HMB.[5][6][11] In healthy individuals, this minor pathway – which involves the conversion of L-leucine to α-KIC and then HMB – is the predominant route of HMB synthesis.[5][6]
A small fraction of L-leucine metabolism – less than 5% in all tissues except the testes where it accounts for about 33% – is initially catalyzed by leucine aminomutase, producing β-leucine, which is subsequently metabolized into β-ketoisocaproate (β-KIC), β-ketoisocaproyl-CoA, and then acetyl-CoA by a series of uncharacterized enzymes.[5][12] HMB could be produced via certain metabolites that are generated along this pathway, but as of 2015 the associated enzymes and reactions involved are not known.[5]
The metabolism of HMB is initially catalyzed by an uncharacterized enzyme which converts it to HMB-CoA.[5][6][9] HMB-CoA is metabolized by either enoyl-CoA hydratase or another uncharacterized enzyme, producing MC-CoA or hydroxymethylglutaryl-CoA (HMG-CoA) respectively.[5][6] MC-CoA is then converted by the enzyme methylcrotonyl-CoA carboxylase to methylglutaconyl-CoA (MG-CoA), which is subsequently converted to HMG-CoA by methylglutaconyl-CoA hydratase.[5][6][12] HMG-CoA is then cleaved into to acetyl-CoA and acetoacetate by HMG-CoA lyase or used in the production of cholesterol via the mevalonate pathway.[5][6]
Effects
Leucine is an mTOR activator. It is a dietary amino acid with the capacity to directly stimulate muscle protein synthesis.[13] As a dietary supplement, leucine has been found to slow the degradation of muscle tissue by increasing the synthesis of muscle proteins in aged rats.[14] However, results of comparative studies are conflicted. Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men.[15] More studies are needed, preferably ones based on an objective, random sample of society. Factors such as lifestyle choices, age, gender, diet, exercise, etc. must be factored into the analyses to isolate the effects of supplemental leucine as a standalone, or if taken with other branched chain amino acids (BCAAs). Until then, dietary supplemental leucine cannot be associated as the prime reason for muscular growth or optimal maintenance for the entire population.
Leucine potently activates the mammalian target of rapamycin kinase that regulates cell growth. Infusion of leucine into the rat brain has been shown to decrease food intake and body weight via activation of the mTOR pathway.[16] Several sensing mechanisms have been proposed; most recently, it has been demonstrated that sestrin 2 can directly bind to leucine and activate mTORC1 activity by promoting its localization to the lysosome.[17][18][19]
Both L-leucine and D-leucine protect mice against seizures.[20] D-leucine also terminates seizures in mice after the onset of seizure activity, at least as effectively as diazepam and without sedative effects.[20] Decreased dietary intake of L-leucine promotes adiposity in mice.[21] High blood levels of leucine are associated with insulin resistance in humans, mice, and rodents.[22]
Safety
Leucine toxicity, as seen in decompensated maple syrup urine disease (MSUD), causes delirium and neurologic compromise, and can be life-threatening.
Excess leucine may be a cause of pellagra, whose main symptoms are "the four D's": diarrhea, dermatitis, dementia and death,[23] though the relationship is unclear.[24]
Leucine at a dose exceeding 500 mg/kg/d was observed with hyperammonemia.[25] As such, the UL for leucine in healthy adult men can be suggested at 500 mg/kg/d or 35 g/d under acute dietary conditions.[25][26]
Dietary sources
| Food | g/100g |
|---|---|
| Soybeans, mature seeds, roasted, salted | 2.868 |
| Hemp seed, hulled | 2.163 |
| Beef, round, top round, separable lean and fat, trimmed to 3 mm (1⁄8 in) fat, select, raw | 1.76 |
| Peanuts | 1.672 |
| Salami, pork | 1.63 |
| Fish, salmon, pink, raw | 1.62 |
| Wheat germ | 1.571 |
| Almonds | 1.488 |
| Chicken, broilers or fryers, thigh, meat only, raw | 1.48 |
| Chicken egg, yolk, raw, fresh | 1.40 |
| Oat | 1.284 |
| soybeans, Edamame, green, raw | 0.926 |
| Beans, pinto, cooked | 0.765 |
| Lentils, cooked | 0.654 |
| Chickpea, cooked | 0.631 |
| Corn, yellow | 0.348 |
| Cow milk, whole, 3.25% milk fat | 0.27 |
| Rice, brown, medium-grain, cooked | 0.191 |
| Milk, human, mature, fluid | 0.10 |
Chemical properties
Leucine is a branched-chain amino acid (BCAA) since it possesses an aliphatic side-chain that is non-linear.
Racemic leucine had been subjected to circularly polarized synchrotron radiation to better understand the origin of biomolecular asymmetry. An enantiomeric enhancement of 2.6% had been induced, indicating a possible photochemical origin of biomolecules' homochirality.[28]
Other uses
As a food additive, L-leucine has E number E641 and is classified as a flavor enhancer.[29]
See also
- Leucines, the isomers and derivatives of leucine
References
- ↑ Dawson, R.M.C., et al., Data for Biochemical Research, Oxford, Clarendon Press, 1959.
- ↑ J. Rosenthal, et al. Department of Medicine, University of Toronto, Toronto, Canada. "Metabolic fate of leucine: A significant sterol precursor in adipose tissue and muscle". American Journal of Physiology Vol. 226, No. 2, p. 411-418. Retrieved 25 March 2008.
- ↑ Nelson, D. L.; Cox, M. M. "Lehninger, Principles of Biochemistry" 3rd Ed. Worth Publishing: New York, 2000. ISBN 1-57259-153-6.
- 1 2 Wilson JM, Fitschen PJ, Campbell B, Wilson GJ, Zanchi N, Taylor L, Wilborn C, Kalman DS, Stout JR, Hoffman JR, Ziegenfuss TN, Lopez HL, Kreider RB, Smith-Ryan AE, Antonio J (February 2013). "International Society of Sports Nutrition Position Stand: beta-hydroxy-beta-methylbutyrate (HMB)". J. Int. Soc. Sports. Nutr. 10 (1): 6. doi:10.1186/1550-2783-10-6. PMC 3568064
 . PMID 23374455.
. PMID 23374455. - 1 2 3 4 5 6 7 8 9 10 11 12 13 14 15 Kohlmeier M (2015). "Leucine". Nutrient Metabolism: Structures, Functions, and Genes (2nd ed.). Academic Press. pp. 385–388. ISBN 9780123877840. Retrieved 6 June 2016.
Figure 8.57: Metabolism of L-leucine
- ↑ Brioche T, Pagano AF, Py G, Chopard A (April 2016). "Muscle wasting and aging: Experimental models, fatty infiltrations, and prevention". Mol. Aspects Med. doi:10.1016/j.mam.2016.04.006. PMID 27106402.
In conclusion, HMB treatment clearly appears to be a safe potent strategy against sarcopenia, and more generally against muscle wasting, because HMB improves muscle mass, muscle strength, and physical performance. It seems that HMB is able to act on three of the four major mechanisms involved in muscle deconditioning (protein turnover, apoptosis, and the regenerative process), whereas it is hypothesized to strongly affect the fourth (mitochondrial dynamics and functions). Moreover, HMB is cheap (~30– 50 US dollars per month at 3 g per day) and may prevent osteopenia (Bruckbauer and Zemel, 2013; Tatara, 2009; Tatara et al., 2007, 2008, 2012) and decrease cardiovascular risks (Nissen et al., 2000). For all these reasons, HMB should be routinely used in muscle-wasting conditions especially in aged people. ... 3 g of CaHMB taken three times a day (1 g each time) is the optimal posology, which allows for continual bioavailability of HMB in the body (Wilson et al., 2013).
- ↑ "KEGG Reaction: R04137". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Retrieved 24 June 2016.
- 1 2 "KEGG Reaction: R10759". Kyoto Encyclopedia of Genes and Genomes. Kanehisa Laboratories. Retrieved 24 June 2016.
- 1 2 Mock DM, Stratton SL, Horvath TD, Bogusiewicz A, Matthews NI, Henrich CL, Dawson AM, Spencer HJ, Owen SN, Boysen G, Moran JH (November 2011). "Urinary excretion of 3-hydroxyisovaleric acid and 3-hydroxyisovaleryl carnitine increases in response to a leucine challenge in marginally biotin-deficient humans". J. Nutr. 141 (11): 1925–1930. doi:10.3945/jn.111.146126. PMC 3192457
 . PMID 21918059.
. PMID 21918059. Reduced activity of MCC impairs catalysis of an essential step in the mitochondrial catabolism of the BCAA leucine. Metabolic impairment diverts methylcrotonyl CoA to 3-hydroxyisovaleryl CoA in a reaction catalyzed by enoyl-CoA hydratase (22, 23). 3-Hydroxyisovaleryl CoA accumulation can inhibit cellular respiration either directly or via effects on the ratios of acyl CoA:free CoA if further metabolism and detoxification of 3-hydroxyisovaleryl CoA does not occur (22). The transfer to carnitine by 4 carnitine acyl-CoA transferases distributed in subcellular compartments likely serves as an important reservoir for acyl moieties (39–41). 3-Hydroxyisovaleryl CoA is likely detoxified by carnitine acetyltransferase producing 3HIA-carnitine, which is transported across the inner mitochondrial membrane (and hence effectively out of the mitochondria) via carnitine-acylcarnitine translocase (39). 3HIA-carnitine is thought to be either directly deacylated by a hydrolase to 3HIA or to undergo a second CoA exchange to again form 3-hydroxyisovaleryl CoA followed by release of 3HIA and free CoA by a thioesterase.
- ↑ "Homo sapiens: 4-hydroxyphenylpyruvate dioxygenase reaction". MetaCyc. SRI International. 20 August 2012. Retrieved 6 June 2016.
- 1 2 "Leucine metabolism". BRENDA. Technische Universität Braunschweig. Retrieved 8 June 2016.
- ↑ Etzel MR (2004). "Manufacture and use of dairy protein fractions". The Journal of Nutrition. 134 (4): 996S–1002S. PMID 15051860.
- ↑ L. Combaret, et al. Human Nutrition Research Centre of Clermont-Ferrand. "A leucine-supplemented diet restores the defective postprandial inhibition of proteasome-dependent proteolysis in aged rat skeletal muscle". Journal of Physiology Volume 569, issue 2, p. 489-499. Retrieved 25 March 2008.
- ↑ Verhoeven, Suzanne; Vanschoonbeek, Kristof; Verdijk, Lex B.; Koopman, René; Wodzig, Will K.W.H.; Dendale, Paul; van Loon, Luc JC (May 2009). "Long-term leucine supplementation does not increase muscle mass or strength in healthy elderly men". Am J Clin Nutr. 89 (5): 1468–75. doi:10.3945/ajcn.2008.26668. PMID 19321567.
- ↑ Cota D, Proulx K, Smith KA, Kozma SC, Thomas G, Woods SC, Seeley RJ (2006). "Hypothalamic mTOR signaling regulates food intake". Science. 312 (5775): 927–930. doi:10.1126/science.1124147. PMID 16690869.
- ↑ Wolfson, Rachel L.; Chantranupong, Lynne; Saxton, Robert A.; Shen, Kuang; Scaria, Sonia M.; Cantor, Jason R.; Sabatini, David M. (1 January 2016). "Sestrin2 is a leucine sensor for the mTORC1 pathway". Science. 351 (6268): 43–48. doi:10.1126/science.aab2674. ISSN 1095-9203. PMC 4698017
 . PMID 26449471.
. PMID 26449471. - ↑ Saxton, Robert A.; Knockenhauer, Kevin E.; Wolfson, Rachel L.; Chantranupong, Lynne; Pacold, Michael E.; Wang, Tim; Schwartz, Thomas U.; Sabatini, David M. (1 January 2016). "Structural basis for leucine sensing by the Sestrin2-mTORC1 pathway". Science. 351 (6268): 53–58. doi:10.1126/science.aad2087. ISSN 1095-9203. PMC 4698039
 . PMID 26586190.
. PMID 26586190. - ↑ Chantranupong, Lynne; Wolfson, Rachel L.; Orozco, Jose M.; Saxton, Robert A.; Scaria, Sonia M.; Bar-Peled, Liron; Spooner, Eric; Isasa, Marta; Gygi, Steven P. (9 October 2014). "The Sestrins interact with GATOR2 to negatively regulate the amino-acid-sensing pathway upstream of mTORC1". Cell Reports. 9 (1): 1–8. doi:10.1016/j.celrep.2014.09.014. ISSN 2211-1247. PMC 4223866
 . PMID 25263562.
. PMID 25263562. - 1 2 Hartman AL, Santos P, O'Riordan KJ, Stafstrom CE, Marie Hardwick J (2015). "Potent anti-seizure effects of D-leucine". Neurobiology of Disease. 82: 46–53. doi:10.1016/j.nbd.2015.05.013. PMID 26054437. Retrieved 26 November 2015.
- ↑ Fontana, Luigi; Cummings, Nicole E.; Arriola Apelo, Sebastian I.; Neuman, Joshua C.; Kasza, Ildiko; Schmidt, Brian A.; Cava, Edda; Spelta, Francesco; Tosti, Valeria (21 June 2016). "Decreased Consumption of Branched-Chain Amino Acids Improves Metabolic Health". Cell Reports. 16: 520–30. doi:10.1016/j.celrep.2016.05.092. ISSN 2211-1247. PMC 4947548
 . PMID 27346343.
. PMID 27346343. - ↑ Lynch, Christopher J.; Adams, Sean H. (1 December 2014). "Branched-chain amino acids in metabolic signalling and insulin resistance". Nature Reviews. Endocrinology. 10 (12): 723–736. doi:10.1038/nrendo.2014.171. ISSN 1759-5037. PMC 4424797
 . PMID 25287287.
. PMID 25287287. - ↑ Hegyi J, Schwartz R, Hegyi V (2004). "Pellagra: dermatitis, dementia, and diarrhea". Int J Dermatol. 43 (1): 1–5. doi:10.1111/j.1365-4632.2004.01959.x. PMID 14693013.
- ↑ Bapurao S, Krishnaswamy K (1978). "Vitamin B6 nutritional status of pellagrins and their leucine tolerance". Am J Clin Nutr. 31 (5): 819–24. PMID 206127.
- 1 2 Elango R, Chapman K, Rafii M, Ball RO, Pencharz PB (2012). "Determination of the tolerable upper intake level of leucine in acute dietary studies in young men". The American Journal of Clinical Nutrition. 96 (4): 759–67. doi:10.3945/ajcn.111.024471. PMID 22952178. Retrieved 7 December 2015.
A significant increase in blood ammonia concentrations above normal values, plasma leucine concentrations, and urinary leucine excretion were observed with leucine intakes >500 mg · kg⁻¹ · d⁻¹. The oxidation of l-[1-¹³C]-leucine expressed as label tracer oxidation in breath (F¹³CO₂), leucine oxidation, and α-ketoisocaproic acid (KIC) oxidation led to different results: a plateau in F¹³CO₂ observed after 500 mg · kg⁻¹ · d⁻¹, no clear plateau observed in leucine oxidation, and KIC oxidation appearing to plateau after 750 mg · kg⁻¹ · d⁻¹. On the basis of plasma and urinary variables, the UL for leucine in healthy adult men can be suggested at 500 mg · kg⁻¹ · d⁻¹ or ~35 g/d as a cautious estimate under acute dietary conditions.
- ↑ Rasmussen B, Gilbert E, Turki A, Madden K, Elango R (2016). "Determination of the safety of leucine supplementation in healthy elderly men". Amino Acids. 48: 1707–16. doi:10.1007/s00726-016-2241-0. PMID 27138628. Retrieved 6 May 2016.
the upper limit for leucine intake in healthy elderly could be set similar to young men at 500 mg kg-1 day-1 or ~35 g/day for an individual weighing 70 kg
- ↑ National Nutrient Database for Standard Reference. U.S. Department of Agriculture. Retrieved 16 September 2009.
- ↑ Meierhenrich: Amino acids and the asymmetry of life, Springer-Verlag, 2008, ISBN 978-3-540-76885-2.
- ↑ Winter, Ruth (2009). A consumer's dictionary of food additives (7th ed.). New York: Three Rivers Press. ISBN 0307408922.
External links
- Leucine biosynthesis
- Combined ingestion of protein and free leucine with carbohydrate increases postexercise muscle protein synthesis in vivo in male subjects