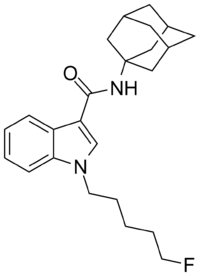
STS-135 (drug)
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | 1354631-26-7 |
| PubChem (CID) | 57404059 |
| ChemSpider | 28189067 |
| Chemical and physical data | |
| Formula | C24H31FN2O |
| Molar mass | 382.51 g/mol |
| 3D model (Jmol) | Interactive image |
| |
| |
STS-135 (N-(adamantan-1-yl)-1-(5-fluoropentyl)-1H-indole-3-carboxamide, also called 5F-APICA) is a designer drug offered by online vendors as a cannabimimetic agent. The structure of STS-135 appears to utilise an understanding of structure-activity relationships within the indole class of cannabimimetics, although its design origins are unclear.[1] STS-135 is the terminally-fluorinated analogue of SDB-001, just as AM-2201 is the terminally-fluorinated analogue of JWH-018, and XLR-11 is the terminally-fluorinated analogue of UR-144. STS-135 acts a potent cannabinoid receptor agonist in vitro, with an EC50 of 51 nM for human CB2 receptors, and 13 nM for human CB1 receptors.[2] STS-135 produces bradycardia and hypothermia in rats at doses of 1–10 mg/kg, suggesting cannabinoid-like activity.[2]
Legal Status
As of October 2015 STS-135 is a controlled substance in China.[3]
Detection
A forensic standard of STS-135 is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[4]
See also
References
- ↑ Wilkinson, S. M.; Banister, S. D.; Kassiou, M. (2015). "Bioisosteric Fluorine in the Clandestine Design of Synthetic Cannabinoids". Australian Journal of Chemistry. 68: 4. doi:10.1071/CH14198.
- 1 2 Banister, S. D.; Stuart, J.; Kevin, R. C.; Edington, A.; Longworth, M.; Wilkinson, S. M.; Beinat, C.; Buchanan, A. S.; Hibbs, D. E.; Glass, M.; Connor, M.; McGregor, I. S.; Kassiou, M. (2015). "Effects of Bioisosteric Fluorine in Synthetic Cannabinoid Designer Drugs JWH-018, AM-2201, UR-144, XLR-11, PB-22, 5F-PB-22, APICA, and STS-135". ACS Chemical Neuroscience: 150508124201002. doi:10.1021/acschemneuro.5b00107.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in Chinese). China Food and Drug Administration. 27 September 2015. Retrieved 1 October 2015.
- ↑ Southern Association of Forensic Scientists