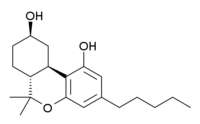
9-nor-9β-Hydroxyhexahydrocannabinol
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | 52171-85-4 |
| PubChem (CID) | 6452587 |
| Chemical and physical data | |
| Formula | C20H30O3 |
| Molar mass | 318.449 g/mol |
| 3D model (Jmol) | Interactive image |
| |
9-nor-9β-Hydroxyhexahydrocannabinol (HHC), is a synthetic cannabinoid derivative which resulted from early modifications to the structure of THC, in a search for the simplest compound that could still fulfil the binding requirements to produce cannabis-like activity.[1][2] HHC is active in its own right with similar potency to THC, but further simplification and variation of this parent structure lead to more potent, yet structurally simpler derivatives such as CP 47,497 and CP 55,940,[3][4][5] which after several steps of modification have become quite structurally distinct from THC, while HHC on the other hand is still substantially similar in structure to THC.
The discovery of this simplified class of cannabinoid derivatives was highly significant in terms of the widespread use of CP 55,940 for early scientific research into the cannabinoid receptors,[6] as well as later work using more complex compounds such as CP 55,244 to map the CB1 binding site in more detail, but aside from these specific applications, these compounds attracted little attention and no compounds from this series were developed for medical use despite favourable safety profiles in animal studies. Unexpectedly, some 25 years later, these compounds came back into prominence when an obscure derivative (C8)-CP 47,497 was found to have been sold as the active ingredient in the "herbal" cannabis substitute product Spice,[7] which ironically has led to a resurgence of interest into laboratory-conducted scientific research of this family of drugs.
See also
References
- ↑ Johnson MR, et al. Potent Analgetics Derived From 9-Nor-9β-Hydroxyhexahydrocannabinol. NIDA Research Monograph 34; 1980. pp 68-74.
- ↑ Melvin LS, Johnson MR. Structure-Activity Relationships of Tricyclic and Nonclassical Bicyclic Cannabinoids. NIDA Research Monograph 79; 1987. pp 31-47.
- ↑ Weissman, A; Milne, GM; Melvin Jr, LS (1982). "Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol". The Journal of Pharmacology and Experimental Therapeutics. 223 (2): 516–23. PMID 6290642.
- ↑ Melvin, LS; et al. (1984). "A cannabinoid derived prototypical analgesic". Journal of Medicinal Chemistry. 27 (1): 67–71. doi:10.1021/jm00367a013. PMID 6690685.
- ↑ Compton, DR; Johnson, MR; Melvin, LS; Martin, BR (1992). "Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents". The Journal of Pharmacology and Experimental Therapeutics. 260 (1): 201–9. PMID 1309872.
- ↑ Devane, WA; et al. (1988). "Determination and characterization of a cannabinoid receptor in rat brain". Molecular Pharmacology. 34 (5): 605–13. PMID 2848184.
- ↑ Auwärter, V; et al. (2009). "'Spice' and other herbal blends: harmless incense or cannabinoid designer drugs?". Journal of mass spectrometry : JMS. 44 (5): 832–7. doi:10.1002/jms.1558. PMID 19189348.