Nickel boride
| Names | |
|---|---|
| Other names
Nickel(II) boride, P−1 catalyst, P−2 catalyst | |
| Identifiers | |
| 12007-01-1 | |
| 3D model (Jmol) | Interactive image |
| ChemSpider | 4891847 |
| ECHA InfoCard | 100.031.345 |
| EC Number | 234-494-6 |
| PubChem | 11389393 |
| |
| |
| Properties | |
| Ni2B | |
| Molar mass | 128.20 g·mol−1 |
| Appearance | black solid |
| Melting point | 1230 |
| insoluble | |
| Hazards | |
| R-phrases | R40, R42/43 |
| S-phrases | S22, S24, S37, S45 |
| Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| | |
| Infobox references | |
Nickel borides are inorganic compounds with the chemical formula NixBy, where x and y vary. A common formula is Ni2B, which is available in two forms, known as P−1 and P−2.[1] Other less common borides of nickel are NiB, Ni3B, o-Ni4B3 and m-Ni4B3 (o for orthogonal, m for metastable).[2]
This article focuses mainly on the most common nickel boride, Ni2B.
Structure and composition
Ni2B has been suggested to be an amorphous composed of nickel bonded to individual boron centres.[3] However it contains nanoparticles of nickel which on heating under inert conditions become more crystaline.[4] The two forms P−1 and P−2 differ in terms of amount of their contamination by NaBO2 adsorbed on the surface. P−1 Ni2B has an oxide to boride ratio of 1:4, whereas that of P−2 Ni2B is 10:1. Their properties differ in terms of catalytic efficiency and substrate specificity.[1]
Preparation
The preparation of amorphous nickel boride is simple compared with other borides which requires high temperatures, special techniques and equipment.[3]
The P−1 form of Ni2B can be generated by mixing nickel(II) sulfate and sodium borohydride in alkaline aqueous solutions. The product is not nickel boride but nanoparticles of nickel disperses in a boron compound matrix.[4] The P−2 form is prepared similarly from Nickel(II) acetate and sodium borohydride in ethanol. The product precipitates as a fine, black amorphous powder. These catalysts were usually generated in situ, which involves the use of NiCl2/NaBH4 mixture system.[1]
Properties
Nickel boride is in the form of black amorphous powder or black granules.[5] It is insoluble in all solvents, but reacts with concentrated mineral acids. The solid is air stable. As expected for a boride, it has a high melting point.[1]
Applications
Ni2B is an efficient catalyst and reducing agent. It is used as a heterogeneous hydrogenation catalyst.
Catalytic hydrogenation
The catalytic activity of P−1 is insensitive to steric hindrance of side chains on the substrate and thus more active, and seldom affects protecting groups. In contrast, P−2 is very sensitive to steric factors.[1] For these reasons, P−1 is usually used for the complete reduction of unsaturated hydrocarbons under mild conditions, while P−2 is useful in partial reductions such as converting alkynes to alkenes in high yields:[6]
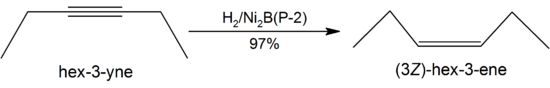
The H2/Ni2B system will not hydrogenolyse ethers, alcohols, aldehydes, amines and amides as it reduces alkenes in preference, even under forcing conditions. It leaves epoxides unaffected, but affects cyclopropanes occasionally. Most esters are stable to Ni2B, except for benzylic, allylic and propargylic esters which are hydrogenlyzed:[1]
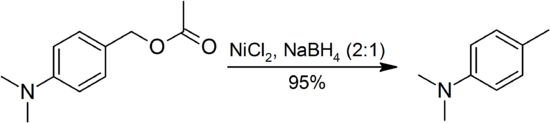
Desulfurization
The NiCl2/NaBH4 system desulfurizes thioamides, thioethers, thioesters, thiols and sulfides. Organic sulfides, disulfides, thiols, and sulfoxides are reduced to hydrocarbons by NiCl2/NaBH4 to hydrocarbons. Illustrated is the reduction of phenothiazine to diphenylamine:
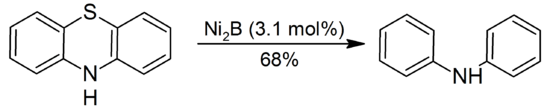
Ni2B can also be used to cleave thioacetals. Since Ni2B is non-pyrophoric, stable in air, and give high yields in many cases, it is proposed as a safer alternative to Raney Nickel for removal of cyclic thioacetals.Desulfurization catalyzed by Ni2B proved to occur with retention of configuration by isotopic labeling.[1]
Reduction of nitrogenous groups
The NiCl2/NaBH4 system reduces aliphatic nitro groups, nitriles and oximes completely to amines. For aryl amines, nitrobenzenes are converted to anilines, and azoxybenzenes to azobenzenes. Azides are cleanly reduced to amines in preference to sterically hindered aliphatic nitro groups:[1]
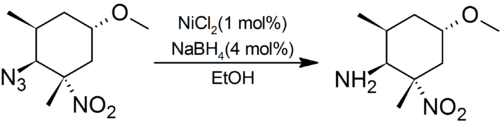
Dehalogenation
Most organic fluorides and chlorides are unaffected by Ni2B, bromides show variable reactivity, and iodides are often completely reduced to hydrocarbons. With Ni2B in DMF, α-bromoketones are reduced to the parent ketones. Vicinal bromides are dehalogenated to alkenes:
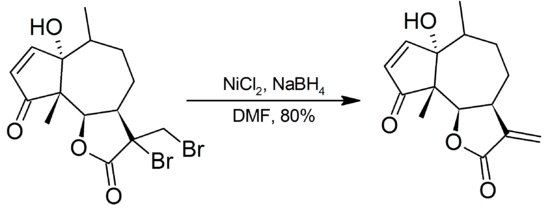
For aryl bromides, the modified system Ni(PPh3)3Cl2/NaBH4 in DMF is used for clean debromination. Reductive cleavage of iodides occurs with retention of configuration.[1]
Safety
Nickel compounds are possible carcinogens and contact with skin should be avoided. Particular care should be taken whenever NiCl2/NaBH4 is used in DMF as sodium borohydride may spontaneously ignite in DMF.
See also
References
- 1 2 3 4 5 6 7 8 9 Steven D. Burke; Rick L. Danheiser (1999). "Nickel boride". Handbook of Reagents for Organic Synthesis, Oxidizing and Reducing Agents. Wiley. p. 246. ISBN 978-0-471-97926-5.
- ↑ Robert A. Scott (2011). "Boron: InorganicChemistry". Encyclopedia of Inorganic Chemistry. Wiley. p. 401. ISBN 9780470862100.
- 1 2 Greenwood, Norman N.; Earnshaw, Alan (1997). Chemistry of the Elements (2nd ed.). Butterworth-Heinemann. p. 147. ISBN 0-08-037941-9.
- 1 2 Template:Cite The unusual nanostructure of nickel–boron catalyst, J. Geng, D.A. Jefferson and B.F.G. Johnson, Chemical Communications, 2007, 969-971. DOI: 10.1039/B615529D
- ↑ Chemicals & Reagents, 2008-2010
- ↑ T. W. Graham Solomons; Craig Fryhle (2007). Organic Chemistry, 9th Edition. Wiley. p. 361. ISBN 978-0-471-68496-1.